Plant Stem Cell and its Pluripotency
Jian Zhu*
Department of Molecular and Cell Biology, School of Life Science and Technology, Tongji University, Shanghai 200092, China
*Address for Correspondence: Jian Zhu, Department of Molecular and Cell Biology, School of Life Science and Technology, Tongji University, Shanghai 200092, China, E-mail: zhujian1@tongji.edu.cn
Submitted: 16 December 2016; Approved: 23 January 2017; Published: 27 January 2017
Citation this article: Zhu J. Plant Stem Cell and its Pluripotency. Int J Stem Cell Res. 2017;3(1): 001-006.
Copyright: © 2017 Zhu J. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
Plant stem cells are special cells that have division and differentiation capabilities and are the initiation cells of tissues, organs, and new plants. Stem cells are reserved at the root tip, stem, and lateral branch, and the growth, development, and reproduction of plants depend on stem cells. The activities of stem cells play an important role in plant life, and their remaining in vivo result in various growth patterns of different plants. The parenchyma cells of plants, a type of stem-like cells, have dedifferentiation capabilities when these cells or tissues are damaged or cultured in vitro. The location, number, state, hormone, and genetic background of stem cells are significant for plant development and reproduction. Their activities are regulated by the expression of several genes, including differentiation, dedifferentiation, and stress genes. This review summarizes the plant stem cell and its pluripotency, focusing on the activities of plant stem cell with hormone and regulatory genes.
Introduction
Plant stem cells are meristem cells with different division capabilities and differentiation degrees. Under different environmental conditions (different hormones), plant stem cells divide and further differentiate into various tissues and organs or form a new plant [1]. Therefore, plant stem cells not only have division and differentiation capabilities but also are the origin of tissues, organs, and new plants.
Distributions of plant stem cells
Plant stem cells distribute in several places of the plant body, such as in the apical meristem that is located in the tips of organs (roots and shoots), and can be divided into two types according to their origin [2]. The protomeristem is located on most tips of roots and shoots. The primary meristem with minimal differentiation degree came from the division of protomeristem and is located close to the protomeristem. The primary meristem can divide and differentiate into various plant tissues, including epidermis, basic tissue, and vascular tissue, which form the root, stem leaf, and flower [3,4].
After initial development, the plant still has many remaining undifferentiated, less differentiated, or not fully differentiated meristems (various types of stem cells), which play an important role in the subsequent growth of the plant. The lateral meristem is an organ existing in the lateral side of the plant organs and is located between the phloem and xylem or near the surface layer of the root and stem. After the complete development of primary plant parts (root, stem, and leaf), these retained stem cells further divide and differentiate for secondary plant growth and thicken the plant, especially some woody plants [5]. Intercalary meristem exists among mature tissues in some plants. Intercalary meristem differentiates into mature tissue but is silently preserved. In a certain development period or certain environmental conditions, such meristem can be further divided and differentiated for plant development, such as the case for bamboo and leek [6]. Meristem (stem cells) retained in the primary plant body (pericycle cells of root, procambium between the phloem and xylem in root, and stem of the primary plant) is the foundation of the subsequent growth of plants.
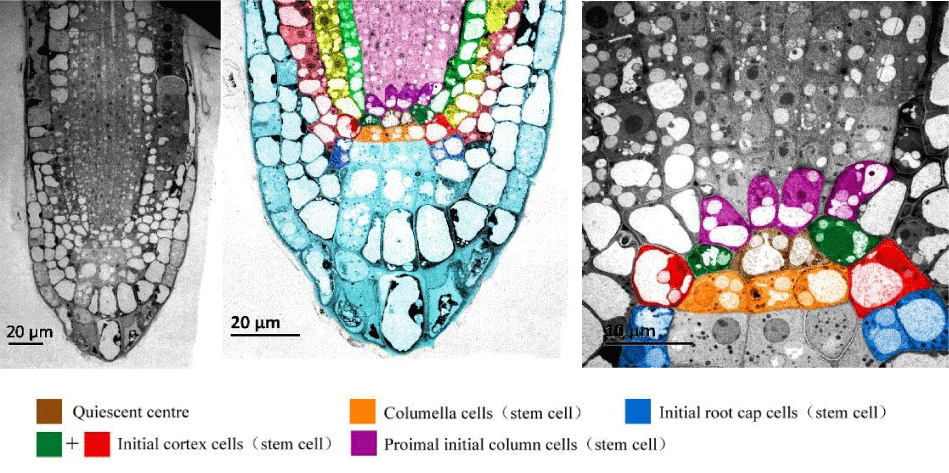
Parenchyma is the largest tissue in plant, which consists of various cells with thin cell walls, large vacuoles, and specific physiological functions, such as cortex, pith, and mesophyll [7]. Parenchyma cells also have division potential. Under special stimulation (hormone or be taken off the cell wall) of wound or in culture, they will return to their original state and dedifferentiate to form a callus that can further form a new plant [8]. The first initiated cell is typically the less differentiated stem cell retained, which will induce the dedifferentiation of adjacent parenchyma during tissue culture [9]. However, the parenchyma, with its dividing potency in plant, only displays its dividing function in a special environment. In addition, the parenchyma is a stem-like cell that is different from stem cells with no or less differentiated state in plant [10]. Some stem cells at the root tip of Arabidopsis thaliana, which are significant for the origin of root tissues, are shown in (Figure 1).
Function of plant stem cells
The activity of plant stem cell is inseparable with the way the plant grows and develops and is gradually formed in long-term evolution. Apart from apical, lateral, and intercalary growth, the branch-growing pattern is also established by the activities of plant stem cell [11]. Plants employ sexual reproduction to adapt to the changes in environmental conditions [12]. Aside from this, plants also employ asexual reproduction to sustain their stable genetic features. Higher plants use many types of asexual reproduction in their long-term evolution; asexual reproduction replaces or coexists with sexual reproduction. Specifically, plants breed differently compared with higher animals. This development results in the generation of numerous stem cells in vivo that have capability or potential for cell division (parenchyma). Under certain conditions, these retained cells can be further divided into a new plant (asexual reproduction) [13]. Asexual reproduction can hence exist in higher plants. In animals, some cancers from stem cells are similar to the rudiment of asexual reproduction in plants [14]. Many stem cells distribute in definite places in plants and initiate at different periods to maintain tip growth, lateral growth, intercalary growth, and asexual reproduction. The growth manner of higher plants provides sufficient production as food for animals and may also be the reason for pluripotency in higher plants.
Plant stem cells in asexual reproduction
The nature of asexual reproduction is to keep the characteristics of species in a relatively stable environment. Asexual reproduction is formed in long-term evolution and is another way of reproduction aside from sexual reproduction [15]. Several plants, such as potato, sweet potato, bamboo, and lotus, have multiple ways of reproducing asexually. Three species of Crassulaceae, Kalanchoe daigremontiana, Graptopetalum paraguayense, and Crassula portulacea are investigated to determine the different types of asexual reproduction owing to the different types of stem cells retained in plants [16].
K. daigremontiana asexually reproduce by producing viviparity seedlings [17]. Some initiation cells are located in the nicks of mature leaf edges and can divide and differentiate into viviparity seedlings (Figure 2) [16,18]. The young plants of the other two species came from a leaf wound. However, the young plants of G. paraguayense are only produced from the leaf basis (Figure 3A-D), where several layers of meristem cells are located; the adventitious buds of C. portulacea are produced by the activities of remained meristems (procambium) in the vascular bundle at any leaf wound (Figure 3E-I). The retained meristem (stem cells) can thus serve as the foundation of plant asexual reproduction [16,19].
Activity of plant stem cells and hormones
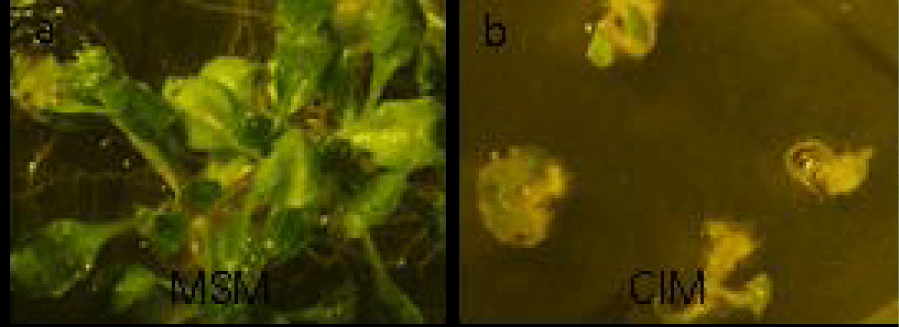
The growth, development, blossoming, and fruit bearing of plants are associated with hormones [20]. The hormones stimulate the remaining stem cells to divide and induce the adjacent potential parenchyma cells to form callus [21]. Callus formation is closely related to the number of stem cells of explants and hormone proportions. The Callus-Inducing Medium (CIM) has a auxin to phytokinkin ratio of 8:1 [22], and different hormone ratios are needed for culturing different plants and explants. The petiole of Arabidopsis with different hormone ratios is investigated, and auxin is found to be responsible for inducing the stem cells and the dedifferentiation of parenchyma cells, whereas the phytokinin accelerates such cell division [23,24].
Hormone ratio is critical, and some genes respond to specific hormones. The cells in almost tranquil state will be stimulated and enter into cell cycles [25]. The interaction between the auxin and cytokinin in root during embryogenesis and the expression of hormone-responsible genes has been proven [26]. The hormone levels and the interaction among them on some specific gene promoters result in expression change and eventually facilitate callus formation [27].
Activity of plant stem cells and relative genes
Different genes regulate various physiological activities [28]. Dedifferentiation is a complex process related to the expression and regulation of specific genes [10]. A few studies have focused on the relationships among genes and their dedifferentiation [29]; however, the corresponding mechanism remains unclear.
The previous results from my lab colleagues show that the remaining stem cells in vascular bundles divide first, and then callus is formed after petiole explants culturing for 24 h to 36 h [9,24]. In a recent study, Affymetrix Gene Chips are used to screen some related genes [30]. The glutathione S transferase induced by hormone is highly expressed in the dedifferentiation process of petiole culturing [30]. WUSCHEL and no-apical-meristem gene family are also highly expressed in the petiole dedifferentiation process [30]. Many members of the Lateral organ Boundary-Domain (LBD) gene family are involved in the dedifferentiation process [30], and the LBD contains genes found in rice ADVENTITIOUS ROOTLESS1. LBD participates in hormonal regulation and combines the other proteins in pericycle cell dedifferentiation, which adjust the starting cell differentiation [31]. Although the types and proportions of stem cells in various explants are different, most of the explants produce similar tissues named calluses; from them, embryogenic calluses (embryoid body predecessor) further differentiate to form young plants. Gene expressions in various stem cells and cell differentiation vary, but their final development is consistent (callus formation and regeneration seedlings) when explants are placed under a special hormone environment (CIM). Different types of stem cells with various differentiation degrees and the expression of their genes are inhibited by stress genes in explants dedifferentiation, thus leading to callus formation. The nature of stem cell differentiation is decided by heredity background and differentiation state [32]. If the cells will become less differentiated or become undifferentiated under the dedifferentiation process, then their differentiation-related genes must be inhibited. Stress genes therefore play an important role in the dedifferentiation process.
The seeds of A. thaliana have a strong differentiated capability to form seedlings when the seeds are cultured on Murashige and Skoog (MS) medium. By contrast, differentiated genes are inhibited by the up-regulation of stress genes when the seeds are cultured in CIM. The seedlings also have smaller sizes and grow slower than those on the MS and form cup-like leaves (Figure 4).
Different genes are involved in the different physical and developmental processes of different plants or explants, including dedifferentiation and pluripotency; they include the genes of hormone response, stress, stem cell maintenance, differentiation, and dedifferentiation; for instance, LBD29 and ARF17 are up-regulated dramatically in dedifferentiation, and WUSCHEL and BBM are involved in differentiation [33-35].
WIND 1 in Arabidopsis has recently been reported to be a gene of dedifferentiation activity in the induced pathway of wounding [36], and a similar homologous gene (WIND 1-like) is also found in Tellungiella halophila [37]. Many genes regulate one phenomenon by different pathways, and they coordinate with one another. Gene expression balance can decide the direction of specific differentiation and even the cell fate. The balance facilitates the realization of plant growth, development, asexual reproduction, and pluripotency. Such facilitation is named “seesaw model” theory, in which the reprogramming of animal cells is affected by the interactions among the genes and their balan cing [38].
Conclusion
Nearly all types of plant growth include primary growth and secondary growth, and sexual and asexual growth originates from the activities of plant stem cell. The success of culturing for plant cells and tissues in vitro is a result of the callus formed from the retained stem cell and stem-like cell, such as parenchyma. Higher plants with tips of root and shoot develop via branch growth. Some perennial plants with periodic growth always keep a certain amount of original stem cells (including undifferentiated and less differentiated protomeristem, primary meristem, and secondary meristem). The presence of these primitive cells and parenchyma is the basis of plant growth, development, sexual or asexual reproduction, and tissue culture. Primitive cells and parenchyma are necessary for plant stem cell activities in various physiological functions, even if they are retained for the next development, which is determined by the status of plant as the first producer in the biological kingdom.
The stem cells of plant and explants under hormone environment differentiate to the callus direction and can be further differentiated to form into a new plant. Whether in vivo and in vitro, the direction of cell differentiation can be influenced by genetic background and environmental hormone. Such a process is complex for plant stem cells activated by hormone signal transduction by considerable gene expression and regulation, eventually resulting in a new plant. A few plants or species are difficult to subject to culturing (to produce less callus or no callus and directly from seedlings). For example, the Chinese orchid, with its protocorm and regeneration seedling produced by the culture of shoot tip, is the elongation of asexual reproductive organs (subterraneous root) [39].
To sum up, the activities of plant stem cells determine the growth and development of plants, and the retaining plant stem cells in vivo result in different ways of plant growth and reproduction in different plants. The number, state, hormonal environment, position, and relative gene expression of plant stem cells and the large amount of parenchyma in plant are the key to plant pluripotency.
Acknowledgments
This paper was supported by a grant from the Natural Science Foundation of China. (Grant No. 31370214).
- Galinha C, Bilsborough G, Tsiantis M. Hormonal input in plant meristems: A balancing act. Semin Cell Dev Biol. 2009; 20: 1149-1156.
- Scheres B, Wolkenfelt H, Willemsen V, Terlouw M, Lawson E, Dean C, et al. Embryonic origin of the Arabidopsis primary root and root meristem initials. Development. 1994; 120: 2475-2487.
- Aichinger E, Kornet N, Friedrich T, Laux T. Plant stem cell niches. Annu Rev Plant Biol. 2012; 63: 615-636.
- Sozzani R, Iyer-Pascuzzi A. Postembryonic control of root meristem growth and development. Curr Opin Plant Biol. 2014; 17: 7-12.
- Jura-Morawiec J, Tulik M, Iqbal M. Lateral Meristems Responsible for Secondary Growth of the Monocotyledons: A Survey of the State of the Art. Bot Rev. 2015; 81: 150-161.
- Beveridge CA, Mathesius U, Rose RJ, Gresshoff P.M. Common regulatory themes in meristem development and whole-plant homeostasis. Curr Opin Plant Biol. 2007; 10: 44-51.
- Secchi F, Pagliarani C, Zwieniecki M.A. The functional role of xylem parenchyma cells and aquaporins during recovery from severe water stress. Plant Cell Environ. 2016.
- Sandhya Srikanth, Tsui Wei Choong, An Yan, Jie He, Zhong Chen. An Efficient Method for Adventitious Root Induction from Stem Segments of Brassica Species. Front Plant Sci. 2016; 7: 943.
- Yu Y, Feng Z, Wang G, Li F, Du X, Zhu J. Initiation of dedifferentiation and structural changes in in vitro cultured petiole of Arabidopsis thaliana. Protoplasma. 2010; 241: 75-81.
- Fangwei Jiang, Zhenhua Feng, Hailiang Liu, Jian Zhu. Involvement of Plant Stem Cells or Stem Cell-Like Cells in Dedifferentiation. Frontiers in Plant Science.2015; 6: 1028.
- Shi B, Zhang C, Tian C, Wang J, Wang Q, Xu T, et al. Two-Step Regulation of a Meristematic Cell Population Acting in Shoot Branching in Arabidopsis. PLoS Genetics. 2016; 12: e1006168.
- Zinn K.E, Tunc-Ozdemir M, Harper J.F. Temperature stress and plant sexual reproduction: uncovering the weakest links. Journal of Experimental Botany. 2010; 61: 1959-1968.
- Burian A, Barbier de Reuille P, Kuhlemeier C. Patterns of Stem Cell Divisions Contribute to Plant Longevity. Curr Biol. 2016; 26: 1385-1394.
- Heidstra R, Sabatini S. Plant and animal stem cells: similar yet different. Nat Rev Mol Cell Biol. 2014; 15: 301-312.
- Schranz ME, Kantama L, de Jong H, Mitchell-Olds T. Asexual reproduction in a close relative of Arabidopsis: a genetic investigation of apomixis in Boechera (Brassicaceae). New Phytol. 2006; 171: 425-438.
- Guo J, Liu H, He Y, Cui X, Du X, Zhu J. Origination of asexual plantlets in three species of Crassulaceae. Protoplasma. 2015; 252: 591-603.
- Batygina T.B. Sexual and asexual processes in reproductive systems of flowering plants. Acta Biologica Cracoviensia. Series Botanica. 2005; 47: 51-60.
- Garcês HM, Champagne CE, Townsley BT, Park S, Malhó R, Pedroso MC, et al. Evolution of asexual reproduction in leaves of the genus Kalanchoe. Proc Natl Acad Sci USA. 2007; 104: 15578-15583.
- Murashige T. Plant Propagation through Tissue Cultures. Annual Review of Plant Physiology. 1974; 25:135-166.
- Bhalerao R.P, Fischer U. Environmental and hormonal control of cambial stem cell dynamics. J Exp Bot. 2017; 68: 79-87.
- Motte H, Vereecke D, Geelen D, Werbrouck S. The molecular path to in vitro shoot regeneration. Biotechnol Adv. 2014; 32: 107-121.
- Banno H, Ikeda Y, Niu QW, Chua NH. Over expression of Arabidopsis ESR1 induces initiation of shoot regeneration. Plant Cell. 2001; 13: 2609-2618.
- Muller B, Sheen J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature. 2008; 453: 1094-1097.
- Li F, Cui X, Feng Z, Du X, Zhu J. The effect of 2, 4-D and kinetin on dedifferentiation of petiole cells in Arabidopsis thaliana. Biologia Plantarum. 2012; 56: 121-125.
- Zhao XY, Su YH, Cheng ZJ, Zhang XS. Cell fate switch during in vitro plant organogenesis. J Integr Plant Biol. 2008; 50: 816-824.
- Xu L, Huang H. Genetic and epigenetic controls of plant regeneration. Curr Top Dev Biol. 2014; 108: 1-33.
- Li W, Liu H, Cheng ZJ, Su YH, Han HN, Zhang Y, et al. DNA methylation and histone modifications regulate de novo shoot regeneration in Arabidopsis by modulating WUSCHEL expression and auxin signaling. PLoS Genet. 2011; 7: e1002243.
- Neelakandan A.K, Wang K. Recent progress in the understanding of tissue culture-induced genome level changes in plants and potential applications. Plant Cell Rep. 2012; 31: 597-620.
- María Berdasco, Rubén Alcázar, María Victoria García-Ortiz, Esteban Ballestar, Agustín F. Fernández, Teresa Roldán-Arjona, et al. Promoter DNA hypermethylation and gene repression in undifferentiated Arabidopsis cells. PLoS One. 2008; 3: e3306.
- Hai-liang Liu, Guang-Chao Wang, Zhenhua Feng, Jian Zhu. Screening of genes associated with dedifferentiation and effect of LBD29 on pericycle cells in Arabidopsis thaliana. Plant Growth Regulation. 2010; 62: 127-136.
- Liu H, Wang S, Yu X, Yu J, He X, Zhang S, et al. ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J. 2005; 43: 47-56.
- Doe C.Q. Neural stem cells: balancing self-renewal with differentiation. Development. 2008; 135: 1575-1587.
- Zhenhua Feng, Xudong Sun, Guangchao Wang, Hailiang Liu, Jian Zhu. LBD29 regulates the cell cycle progression in response to auxin during lateral root formation in Arabidopsis thaliana. Ann Bot. 2012; 110: 1-10.
- Mayer KF, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell. 1998; 95: 805-815.
- Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, et al. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell. 2002; 14: 1737-1749.
- Iwase A, Mitsuda N, Koyama T, Hiratsu K, Kojima M, Arai T, et al. The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr Biol. 2011; 21: 508-514.
- Zhou Cheng, Guo Jiansheng, Feng Zhenhua, Cui Xianghuan, Zhu Jian. Molecular characterization of a novel AP2 transcription factor ThWIND1-L from Thellungiella halophila. Plant Cell, Tissue and Organ Culture (PCTOC). 2012; 110: 423-433.
- Shu J, Wu C, Wu Y, Li Z, Shao S, Zhao W, et al. Induction of pluripotency in mouse somatic cells with lineage specifiers. Cell. 2013; 153: 963-975.
- Zhu J, ZJ, Shi H, Xie L, Study of morphology and anatomy on the protocorm in tissue culturing of Cymbidium sinense. J. of South China Agri.Univ. 2000; 4: p. 47-50.



Sign up for Article Alerts