An Introduction to Experimental Design for Developing Product and Process Design with Focusing on Box-Behnken Design?
Jyoti Gupta1* and Ved Prakash2
1Assistant Professor HIMT College of Pharmacy, India
2Associate Scientist at Novonordisk Denmark
*Address for Correspondence: Jyoti Gupta, Assistant Professor HIMT College of Pharmacy, India, Tel: +91-9899922991; E-mail: jyogupta21@gmail.com
Submitted: 02 August 2020; Approved: 07 August 2020; Published: 26 August 2020
Citation this article: Gupta J, Prakash V. An Introduction to Experimental Design for Developing Product and Process Design with Focusing on Box-Behnken Design. Int J Pharma Anal Acta. 2020;3(1): 030-037.
Copyright: © 2020 Gupta J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Design of experiments; Development of a design; Approach to design of experiment
Download Fulltext PDF
One of the main issue occurs in developing a pharmaceutical product is increasing experiment time and cost of operation. The quality of product and method depends upon many input factors. With a promising experimental planning it becomes easier to manage input factors and finally one can achieve a desired output. Design of Experiment (DOE) is an effective and easiest statistical tool which is used to analyze process and manage input factors. This is done by selecting dependent and independent variables at different levels. Many DOE software are using to enforce better control over the outcomes. Box- Behnken design is a type of DOE which is used for response surface methodology. This paper seeks some aspects of applications, measuring and discussing contemporary methodology of DOE with special reference to Box-Behnken design. It is a systematic approach to identify that how can dependent parameters affect the response variable or product performance.
Introduction
APIs and excipients usually can create issues with performance in equivalence. A well-defined excipients and processes are required for manufacturing of a quality pharmaceutical product. As a result, selection of performance tests is required for understanding the excipients role in the relevant dosage form. Hence, performance and identification of functional characteristics of excipients are significant aspects for quality, development, manufacturing, and performance of dosage forms. Thus a complete understanding of excipients properties requires suitable selection of tests and performance [1].
DOE is a well-organized, statistical methodology that determines the importance of specific variable and how to optimize performance. To study the effect of individual factor and their interaction on response in DOE, the levels of factors changed simultaneously. The input factor can be concentration of excipients, load, methodology of formulation, temperature etc. The effect of variable on response is analyzed by comparing response and carrying repeated experiments at same level [1-4].
The development of DOE was first implemented by Ronald Fischer in 1935in agriculture in which he described three principles of randomization, replication and blocking which is widely used in DOE. Then Box and Wilson in 1950 bring the concept of response surface methodology in 1950 Genichi Taguchi introduced Taguchi method which popularized DOE in many industries [5,6].
There is a vast literature and contributor in field of DOE. Now a day’s development and optimization of formulation are carrying by using DOE. DOE is gaining importance and many studies approaches DOE in formulation. These are some literature to illustrate the contributions that have been carried out in the field of DOE. Furosemide nanosuspension and Clotrinazole nanoemulsion was developed and optimized by using DOE [7,8]. Novel glycosaminoglycan based injectable formulation was developed by using DOE [9]. A multidose Propofol nanoemulsion was formulated and further evaluated by using full factorial design [10]. There are several approaches carried out in the field of DOE [7-10].
What is Design of Experiments?
“DOE is a statistical tool which determine relationship between the factored affecting the process and output of that process. DOE is associated with planning, conducting and analyzing outcomes by evaluating precondition parameters which is represented as independent variable or input variable. Generally the outcomes is hypothesized that one or more input variable is affecting one or more independent variable or output variable. The response gets systematically changed according to the design matrix obtained from input variables [5-6].
In a DOE approach, formulation and process parameters that is exactly related to the Critical Quality Aspects (CQA) of the final dosage form. It means that input parameters are responsible for the variations in the end product qualities. The combination and interaction of all input parameters (e.g. material attribute) and various process parameters which potential and critically affects final product quality is termed as the design space [5-8].
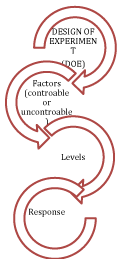
Component of DOE (Figure 1) is a systematic representation that emphasized understanding of aspects of process that are analyzed by DOE. Factors can be either controllable or uncontrollable. The controllable factors are the parameters that can be modified within the experiment. Uncontrollable factors are the parameters that cannot be changed within the experiment. Levels are a different factors value that runs an experiment. Each runs of an experiment carry a combination of levels of the factors. Response is the outcome that has desired effect [11,12].
Objectives of the Design of Experiments [12-15]
To determine which variables are most prominent on the responses,
➣ To determine the responses that is almost near to the desired target values,
➣ To set the experiment to facilitate the variability in the responses became small
➣ To determine or minimize uncontrollable variables on the responses.
Advantages of DOE over other Methods [11-13]
➣ Provide detailed experimental plan for doing experiment.
➣ More information per experiment with improved efficiency.
➣ Well-organised and easiest approach.
➣ Information consistency.
➣ Ability to examine interactions and more reliable prediction.
➣ Less batch failure.
➣ Validation approach with innovative process.
➣ Reduced testing and improved yields.
➣ Lead closer to target requirements.
Context of Development of a Design
Implementation of DOE in pharmaceutical development will not only assist modernization of chemistry but also assist in manufacturing and control new drug and Abbreviated New Drug Applications (ANDAs) for estimation of pharmaceutical quality. The implementation of DOE for pharmaceutical development and production is a part of authentic and practical implementation of some core concepts and principles well-defined by the FDA’s pharmaceutical cGMP’s [14].
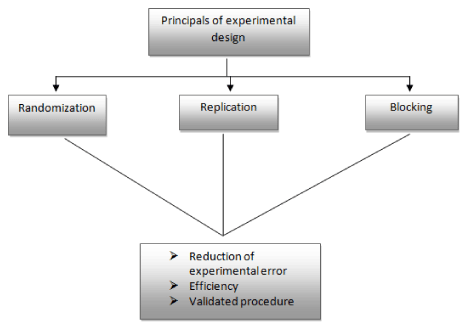
Development of the experimental design is based on three basic principle (Figure 2) i.e. randomization, replication and blocking. The application of DOE and other design techniques is now spread over in the industries process, also it is widely used in research and development work.
Experimental design is analyzed by using softwares like Design-Expert software and Taguchi software. Taguchi recommended highly fractionated factorial designs and statistical methods to explain the various problems [15-17].
Criteria for Statistical Design [12,13,15]
➣ Concern of Objectives
• Nature of predicted conclusions
• Description of concepts
• Determination of observable variables
➣ Factor Effects
• Purging of systematic error
• Identification of relationships
• Consideration of entire experimental section
➣ Efficiency
• Multiple factors
• Screening experiments
• Fractional factorials
➣ Randomization
Determination of observable variables is greatly influenced by both the experimental design and the assortment of information on uncontrollable factors. When assessing factor effects it is important to explore the entire experimental region of interest. The combinations of factor levels used in a statistical design should be selected to fill out the experimental region. If a factor is only studied over a narrow portion of the experimental region, important effects may go undetected.
Randomization can help ensure that all levels of a factor have an equal chance of being affected by process. Randomization affords protection from bias by tending to average the bias effects over all levels of the factors in the experiment.
Types of Design [13-15]
Types of designs which are mainly used are as follows:
➣ Simplex design
➣ Full factorial design
➣ Response surface design
➣ Fractional factorial design
➣ Placket-burman design
➣ Box-Behnken design
➣ Mixture design
Methodology for DOE [11,12,17]
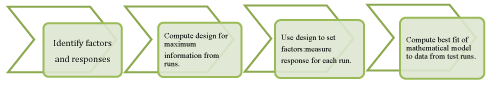
A well designed experiment for obtaining good outcomes requires selection and identification of dependent and independent variable. Select a choice of experimental design that achieves the objective of the experiment. Then identify the factors and levels to be investigated. Execute the design by using suitable software. Analyze and interpret the DOE data obtained from the runs and computed with mathematical equation. Once we know about the variable factor that affect the response, changes in the objective or variable may pursue.
Box-Behnken Design [18-20]
Box-Behnken design is an experimental designs used for response surface methodology which consists of a set of points arranged at the midpoint of each edge and the replicated centre point of multi-dimensional cube. For designing the experiment independent variables and dependent variables are selected and executed. A Box-Behnken design with two factors does not exist. A Box-Behnken design requires three factors (input parameters) and three levels. Selected independent variable is placed at three levels values i.e. minimum, optimum and maximum coded as -1, 0, +1. A Box-Behnken design is considered to be more skilled designed compare to other three level full factorial design. It cannot be applied to study the factors having more than three levels [12,16,22,23].
The polynomial equation produced by this experimental design is as follows:
Y1 = b0 + b1X1 + b2X2 + b3X3 + b12X1X2+ b13X1X3 + b23X2X3 +b11X12 + b22X22 + b33X32
WhereY1- dependent variable,
b0- intercept,
b1 to b33- regression coefficients,
X1, X2 and X3- independent variables
The quadratic response surface of experimental design is analyzed by using Design-Expert software, which gives considerable practical information and endorsed the utility of statistical design for carry out the experiments. Polynomial equations with main effect and interaction factors are determined by Design-Expert software. Therefore, optimum values of the variables are determined along with the obtained experimental data by using this software [23,24].
Design of experiments is concerned about factors, responses, a model, and runs. Design expert version 10 software helps you to determine if and how a factor affects a response. Box-Behnken design is suitable for examine the quadratic response surface. It is suitable for the experimenter who wants to collect data and see response surface over a wide ranges of values from different variables it can be easily find a combination of factors that go with specified target. To apply Box-Behnken design need to follow the following steps [12,13,25,26]:
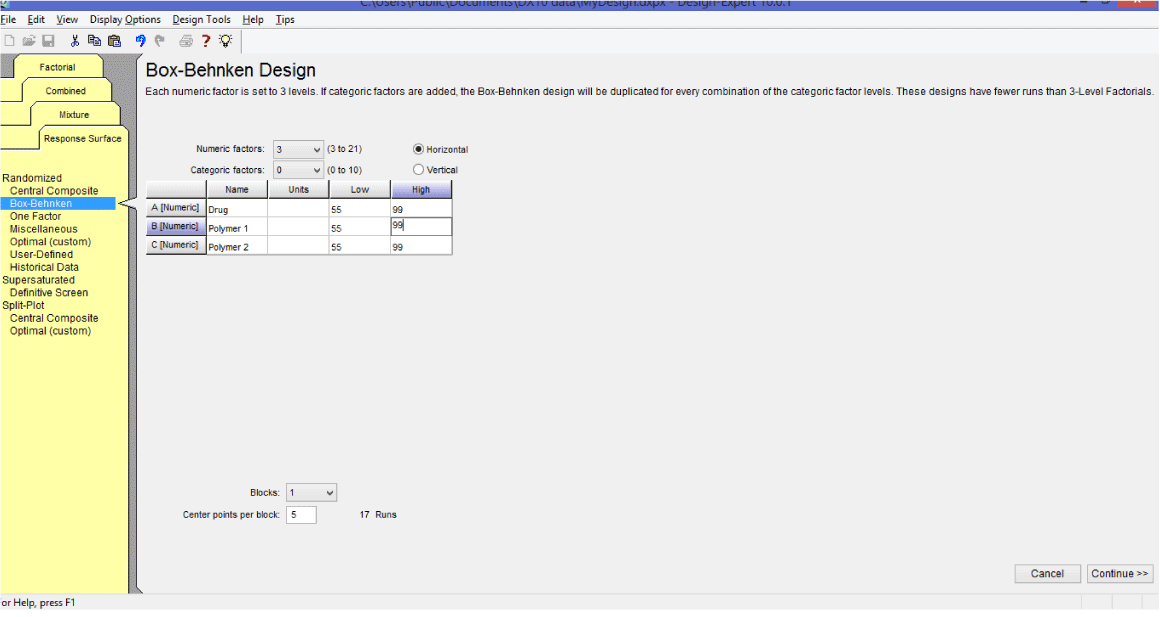
1. First select three factors for example consider drug as (A) and polymers as (B) and (C).
2. Now open design expert version 10 software.
3. Select new design>response surface design.
4. Click on response surface design and select Box-Behnken and feed the name and values of factors.
5. Clicks on continue and then finish.
6. Feed the response obtain from the experiment in column response 1.
This Box-Behnken statistical design gives 17 runs combination for three factors (A, B and C) and three levels (-1, 0 and +1) to construct a polynomial models for the optimization process.
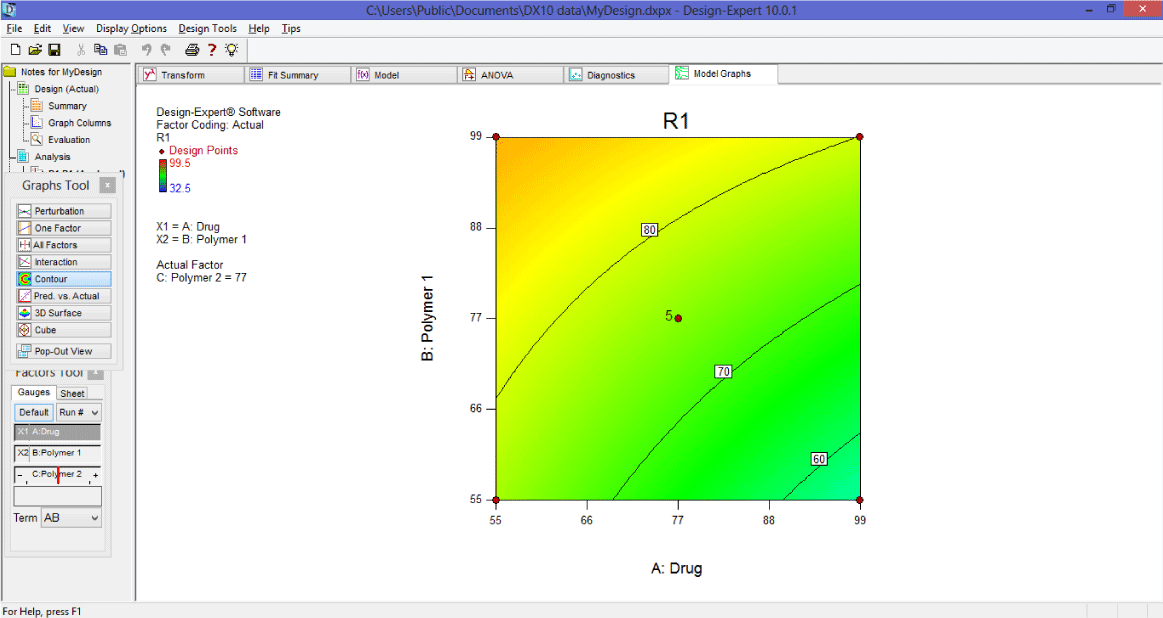
A Response Surface Plot (Contour Profiler) [12-13]
Response surface can also be seen by contour profiler which is useful for examine response surface graphically. It is useful especially when there are multiple responses.
1. With the outcome response contour profiler is obtained by clicking on analysis and select the equation .
2. By clicking on fit summary you can get quadratic equation.
3. Now click on Model graph > contour.
4. Click on 3D surface to view 3D presentation.
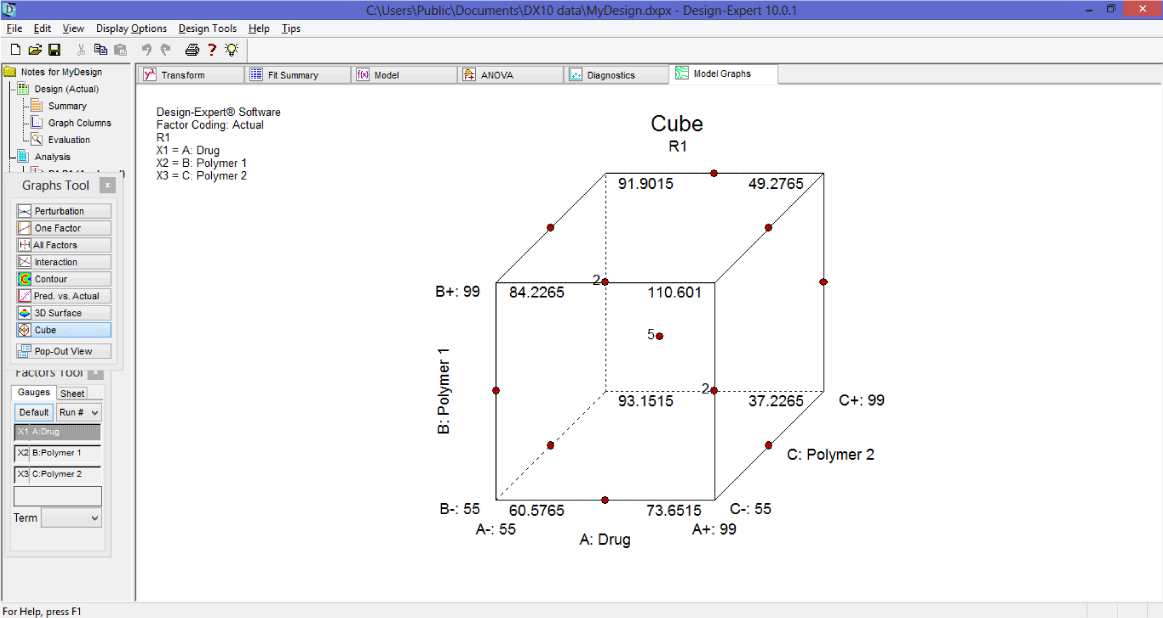
5. Now click on cube to see geometric structure of design with all the three effects on the plot.
The experimental points in Box-Behnken Design (Figure 8) are lie on the hypersphere which is equally distant from central point.
Application of Box-Behnken Design
Numerous studies have been by response surface methodology obtained from Box-Behnken Design in order to optimize the process conditions. Box-Behnken Design have been widely applied in many field like food industry, biochemical processes, construction industry, chemical processes and pharmaceutical industry.
Many researcher have been carried Box-Behnken Design in food industry which involves extraction of oil, pigments, polysaccharide etc by applying response surface methodology to optimize extraction process conditions [24].
Several studies have been done in the field of biochemical processes by optimization of process parameters like temperature, pH, particle size, moisture percentage and incubation period [25].
Box-Behnken Design has applied in construction industry. In a study researcher applied Box-Behnken Design for mixture made with crushed brick and tiles aggregation to optimize the parameter like water cement ratio [26].
Box-Behnken Design found to be useful for optimization of chromatographic analysis. Box-Behnken Design carried on parameters like retention time, tailing factor, peak symmetry, relative retention time number of theoretical plates etc to study the interaction between them [27].
Several studies have been carried out in the formulation of pharmaceutical products. Solid lipid nanoparticles of chloramphenicol was developed and optimized using Box-Behnken Design in order to study the effect of dependent variable like entrapment efficiency, drug loading and turbidity [28].
Conclusion
DOE is an effective and inexpensive approach to determine the precise estimation of interaction between the factors. Nowadays it is widely used due to its advantage over conventional methods. We cannot examine this information with one way analysis or single variable. Box-Behnken design is a response surface methodology that helps in better understanding and optimization of response.
- Catherine S. Understanding the role of excipient functional category & performance-related tests in a quality-by-design fra mework. Drug Development and Delivery. 2012. https://tinyurl.com/yy6sqhv3
- Jun Huang, Goldi Kau, Chunsheng Cai, Ramarao Chatlapalli, Pedro Hernandez-Abad, Krishnendu Ghosh, et al. Quality by design case study: An integrated multivariate approach to drug product and process development. Int J Pharm. 2009; 382: 23-32. DOI: 10.1016/j.ijpharm.2009.07.031
- Versyck KJ, Bernaerts K, Geeraerd AH, Van Impe JF. Introducing optimal experimental design in predictive modeling: A motivating example. Int J food microbiology. 1999; 51: 39-51. DOI: 10.1016/s0168-1605(99)00093-8
- Durugkar S, Kallam M, Ranadive S, Deorkar N. Development of solid dose formulations with highfunctionality excipients using quality by design principle. Pharma Times. 2013; 45: 3. https://tinyurl.com/y2fd47v8
- Sethuramiah A, Kumar R. Statistics and experimental design in perspective. Modeling of Chemical War. 2016; 129-159.
- John R. Design of experiments. Extrusion. 2nd ed. 2014. 291-308.
- Mohammad H Shariare, Mohammad A Altamimi, Akbar L Marzan, Rahnuma Tabassum, Basarat Jahan, Hasan M Reza, et al. In vitro dissolution and bioavailability study of furosemide nanosuspension prepared using Design of Experiment (DoE). Saudi Pharmaceutical Journal. 2019; 27: 96-105. DOI: 10.1016/j.jsps.2018.09.002
- Bhavin Y, Gajera D, Shah A, Dave H. Development of an amorphous nanosuspension by sonoprecipitation-formulation and process optimization using design of experiment methodology. International Journal of Pharmaceutics. 2019; 559, 348-359. DOI: 10.1016/j.ijpharm.2019.01.054
- Marta Cicognani, Silvia Rossi, Gabriele Vecchi, Andrea Maria Giori, Franca Ferrari. DoE-assisted development of a novel glycosaminoglycan-based injectable formulation for viscosupplementation. Pharmaceutics. 2020; 12: 681. DOI: 10.3390/pharmaceutics12070681
- Hota SS, Pattnaik S, Mallick S. Formulation and evaluation of multidose propofol nanoemulsion using statistically designed experiment. Acta Chimica slovenica, 2020; 67. DOI: 10.17344/acsi.2019.5311
- Timothy D, Blackburn PE. An Introduction to QbD (Quality by Design) and Implications for Technical Professionals. ISPE CASA Annual Technology Show. 2011. https://tinyurl.com/y58l3yvb
- Tania P, Deborah J. An annotated bibliography of application papers using certain classes of fractional factorial and related design. Journal of Statistical Planning and Inference. 2002; 106: 245-269. DOI:10.1016/S0378-3758(02)00216-1
- Collins LM, Dziak JJ, Li R. Design of experiments with multiple independent variables: A resource management perspective on complete and reduced factorial designs. Psychol Methods. 2009; 14: 202-224. DOI: 10.1037/a0015826
- Nasr MN. Implimentation of quality by design (QbD): Status challenges, and next steps. Fda Advisory Committee for Pharmaceutical Science. 2006. https://tinyurl.com/y3vm9v5b
- Khar RK, Choudhary V. Application of contemporary concept of Qbd, DOE and Fbd for drug development. The pharma review. 2012; 73-76. https://tinyurl.com/yxfyjhq2
- Edmer M, Dilma B, Carasek E. Application of fractional factorial experimental and box behnken design for optimization of single drop microextraction of 2,4,6-trichloroanisole and 2,4,6-tribromoanisole from wie samples. Journal of chromatography. 2007; 1148: 131-136. DOI: 10.1016/j.chroma.2007.02.079
- Montgomery DC. Experimental design for product and process design and development. The Statistician. 1999, 159-177. https://tinyurl.com/y3jzpyth
- Andrew Prpich, Mary T am Ende, Thomas Katzschner, Veronika Lubczyk, Holger Weyhers, Georg Bernhard. Drug product modeling predictions for scale up of tablet film coating: A quality by design approach. Computers and chemical engineering. 2010; 34: 1092-1097. DOI: 10.1016/j.compchemeng.2010.03.006
- Chivate N, Patil S, Saboji J, Chivate A. A complete review on solid dispersion technology and factorial design. Current Pharma Research. 2012; 659-667. DOI: 10.33786/JCPR.2012.v02i04.011
- Swarbrick J. Encyclopedia of pharmaceutical technology. 1st, 3rd ed. 2452-2466.
- Mason RL, Gunst RF, Hess JL. Statistical design and analysis of experiments. 2nd ed. Willey Publication. 2003.
- Patel NV, Patel JK, Shah SH. Box-Behnken experimental design in the development of pectin-compritol ATO 888 compression coated colon targeted drug delivery of mesalamine. Acta Pharm. 60. 2010; 39-54. DOI: 10.2478/v10007-010-0008-9
- Taha A, Dana E, Hessien M. Evaluation of catalytic and adsorption activity of iron nanoparticles greenly prepared under different conditions: Box–Behnken design. Molecular Simulation. 2020. DOI: 10.1080/08927022.2020.1784475
- Yolmeh, M, Jafari SM. Applications of response surface methodology in the food industry processes. Food and Bioprocess Technology. 2017: 413-433. https://tinyurl.com/y3bo9xyx
- Verma N, Kumar V. Application of Box–Behnken design for the optimization of cellulose production under solid state fermentation. SN applied science. 2019. DOI:10.1007/s42452-019-1779-3
- Ivana Miličević. Application of Box–Behnken design in properties modelling of concrete with crushed brick and roof tiles. 2014. https://tinyurl.com/y46nja5d
- Czyrski A, Sznura J. The application of Box–Behnken design in optimization of HPLC separation of fluoroquinolones. Scientific Reports. 2019. https://tinyurl.com/y4dmu7aq
- Jifu Hao, Xinsheng Fang, Yanfang Zhou, Jianzhu Wang, Fengguang Guo, Fei Li, et al. Development and optimization of solid lipid nanoparticle formulation for ophthalmic delivery of chloramphenicol using a Box-Behnken design. Int J Nanomedicine. 2011; 6: 683-692. DOI: 10.2147/IJN.S17386


Sign up for Article Alerts