Aquaporin 1-Inhibition Promote Angiogenesis in Malignant Glioma: An Intro Study?
Yinsheng Chen1* Ji Zhang1*, Haicheng Xia2, Zhengquan Zhu2# and Jian Wang1#
1Department of Neurosurgery/Neuro-oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
2Department of Neurosurgery, The third affiliated hospital of Xinjiang Medical University, Urumqi, China
*Yinsheng Chen and Ji Zhang contributed equally to this work.
#Correspondence and requests for materials should be addressed to Jian Wang, E-mail: wangjian2@sysucc.org.cn
*Address for Correspondence: Yinsheng Chen, Department of Neurosurgery/Neuro-oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China, E-mail: chenyinsh@sysucc.org.cn
Submitted: 16 November 2017; Approved: 28 November 2017; Published: 30 November 2017
Citation this article: Chen Y, Zhang J, Xia H, Zhu Z, Wang J. Aquaporin 1-Inhibition Promote Angiogenesis in Malignant Glioma: An Intro Study. Sci J Neurol Neurosurg. 2017;3(4): 076-078.
Copyright: © 2017 Chen Y, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Download Fulltext PDF
Malignant glioma is one of the most aggressive and angiogenic human tumors and characterized by microvascular proliferations. Vasculogenic Mimicry (VM) is a newly described phenomenon associated with increased aggressiveness and poor overall survival in glioma. Aquaporin 1 (AQP1) is expressed by normal vascular endothelium and has been found involved in mediating cell motility and proliferation in other malignancies. We investigated the role of AQP1 in angiogenesis in glioma cell line. Our data show that AQP1 promotes VM formation in malignant glioma, it could be a potential target for anti-vascular therapy
Introduction
Glioma are the most frequent and malignant primary brain tumors in adults and have a poor overall survival and quality of life. Despite standard therapy (surgery and conventional radio-chemotherapy), the average survival time is only 15 months, and the best 5-year survival rate reported is only 9.8% [1]. Although malignant glioma shows characteristics of increased angiogenesis, anti-angiogenic therapy has not consistently resulted in a survival advantage in the real world [2]. Maniotis [3], reported that highly aggressive uveal melanomas can form vessels by tumor cells instead of endothelial cells and this phenomenon of tumor vascularization has been named as VM. In other study, VM was described the ability of aggressive tumor cells to form vasculogenic like networks result in their high plasticity [4-6]. In our previous study, VM has been observed in some malignant glioma and predicted poor overall survival [7-9]. However, VM formation and its molecular mechanisms has not been fully explained. AQP1, a water channel protein expressed on cell membranes throughout the body, including vascular endothelial cells [10], has been implicated up-regulation in glioma tissues and cell lines, as in comparison to in normal tissues [11]. Furthermore, increased AQP1 protein and mRNA expression can affect glioma apoptosis and proliferation via regulated CyclinD1 and Bcl-2 [12], which is essential for glioma migration and invasion.
Saadoun et al. reported that AQP1 is important for melanomas angiogenesis, observing reduced micro-vessel density in AQP1-null mice, and found AQP1 expression in tumor vasculature in wild-type mice [13]. Due to its established role in cell migration in glioma cells, and in angiogenesis in other tumor types, we proposed that blockade of AQP1 may inhibit VM formation. The aim of this study was to investigate the potential role of AQP1 in VM of glioma cell lines.
Methods
Cell culture
T98G and U251 glioma cell lines and Human Umbilical Vein Endothelial Cells (HUVECs) were obtained from ATCC (Manassas, VA, USA). HUVECs were used as positive controls in VM formation assays. All cell lines were cultured in complete DMEM, following the procedure described in our previous report [14].
AqB050 Aquaporin Blocker
The AQP1 specific pharmaceutical blocker, AqB050, was obtained from consenting donors. The stock solution (40mM) of AqB050 was prepared in DMSO and diluted to desired concentration for experiments. Previous investigations had confirmed that AqB050 was not cytotoxic to cells at working concentration [15].
Western blot assay
Cell lysates were prepared with cell lysis buffer (Cell signaling, USA). After sonication, centrifugation, and protein assay (Pierce protein assay kit, USA), 50 lg protein and an equal volume of 29 sample buffer (62.5 mmol/L Tris–HCl pH6.8, 2% (w/v) sodium dodecyl sulfate, 10% glycerol, 50 mmol/L dithiothreitol, and 0.01% (w/v) bromophenol blue) were heated at 94℃ for 5 min. Proteins were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and transblotted onto a Polyvinylidene Difluoride (PVDF) transfer membrane (Bio-Rad, USA). The blot was blocked in PBS containing 0.1% Tween-20 and 5% skim milk at 37℃ for 1 h. The membrane was then incubated in primary antibody AQP1 (1:200) at 4℃ overnight, followed by treatment with secondary antibody conjugated with horseradish peroxidase (1:1000). Proteins were visualized using the ECL system (Amersham Biosciences, USA). Data were the mean of triplicate experiments.
In vitro vasculogenic mimicry assay
In vitro VM formation was tested using a three-dimensional culture model [9]. The cells were dissociated and re-suspended at 5×104 cells/mL in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (Hyclone, USA). Wells of 24-well tissue culture plates were coated with Matrigel (0.1 mL/well, BD Biosciences, USA) which was allowed to polymerase at 37℃ for 30 min. The indicated cell suspension was then plated at 0.5 mL/well onto the surface of Matrigel and incubated at 37℃. Cells were photographed using microscope.
Results
AqB050 inhibited the protein expression of AQP1
To verify the inhibition effect of AqB050, western blot arrays was performed. Date show that AqB050 could effectively inhibit protein expression of AQP1 in glioma cell line T98G and U251 (Figure 1).
AQP1 treatment Inhibits VM formation in cell lines In vitro
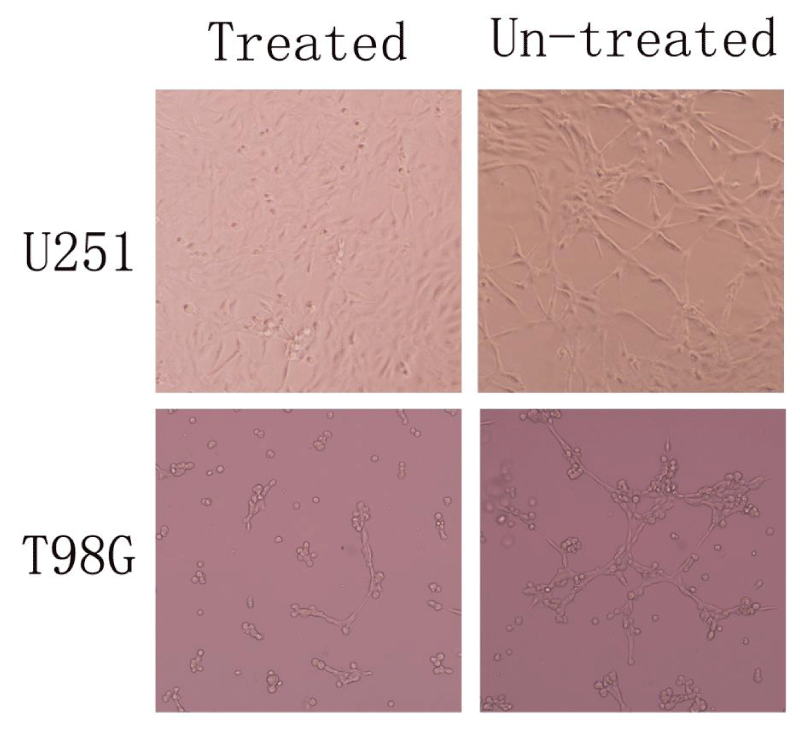
In order to investigate the capacity of different cells to display vasculogenesis In vitro, we used three-dimensional matrigel tube formation assays for direct comparison between un-treated glioma cell lines and AqB050-treated ones. The result show that the un-treated group underwent a dramatic reorganization and formed efficiently a vasculogenic network of tubular structures within 48 h while AqB050-treated group formed fewer structures even after 6 days (Figure 2).
Discussion
Glioma is the most frequent primary brain tumors and characterized by microvascular proliferations. However, anti-angiogenic therapy has shown promising but insufficient efficacy on gliomas [16,17]. The glioma –immune interaction also be studied through mathematical modeling to better understand the underlying dynamics [18,19]. For years, sprouting angiogenesis has been considered an exclusive mechanism of tumor vascularization. In 1999, VM was first reported as a functional tumor microcirculation in melanomas [3]. VM describes a novel mechanism by which highly aggressive tumor cells can form vessel-like structures themselves and may function as blood supply networks, by virtue of their high plasticity [6]. For cancer patients, VM is associated with poor prognosis, as the unique structure of VM channels facilitates tumor cell metastasis [17]. Recent studies suggest that VM occurs in aggressive tumors, including gliomas. Traditional anti-vascular therapies focused on endothelial cells are not effective in blocking tubular network formation by tumor cells. Maybe, successful treatment of gliomas should involve targeting both VM and angiogenesis. However, the detailed molecular mechanisms for VM are not fully understood.
Recently, several genes such as Matrix Metalloproteinase-2 (MMP-2), Membrane Type-1 Matrix Metalloproteinase (MT1-MMP), Vascular Endothelial Growth Factor (VEGF), COX-2, and Vascular Endothelial Cadherin (VE-cadherin) have been implicated in VM channel formation in human tumors, making them potential targets for therapy with anti-sense oligonucleotides or monoclonal antibodies [20]. AQP1 is a water channel protein, some study has confirmed that AQP1 promote glioma proliferation and reduce apoptosis [12]. In this study, AqB050 was used to inhibit protein expression of AQP1 in glioma cell line T98G and U251, we went on to perform a three-dimensional matrigel tube formation assays to investigate the effect of AQP1. We found that the un-treated group underwent a dramatic reorganization and formed efficiently a vasculogenic network of tubular structures within 48 h while AqB050-treated group formed fewer structures even after 6 days. And it turns out that AQP1 may be a key regulatory factor for VM in glioma cells.
Conclusion and Suggestion
In conclusion, we certified by our study that inhibition of AQP1 suppresses VM, in another word, AQP1 which involved in glioma migration and invasion, may also contribute to VM in glioma cells, AQP1 may be a potential target for anti-VM therapy. But we need to know, VM appears to be a result of a highly redundant and complex interaction of different molecules. Clinically, inhibition of tumor progression through limiting blood supply will likely require knockdown of multiple molecular pathways and will be dependent on individual tumor cell responsiveness.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352: 987–996. https://goo.gl/7uE7go
- Field KM, Jordan JT, Wen PY, Rosenthal MA, Reardon DA. Bevacizumab and glioblastoma: scientific review, newly reported updates, and ongoing controversies. Cancer. 2015; 121: 997–1007. https://goo.gl/RQJ5Up
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Peer J, et al. Vascular channel formation by human melanoma cells in vivo and In vitro: vasculogenic mimicry. Am J Pathol. 1999; 155: 739–52. https://goo.gl/Ju9xTQ
- Guzman G, Cotler SJ, Lin AY, Maniotis AJ, Folberg R. A pilot study of vasculogenic mimicry immunohistochemical expression in hepatocellular carcinoma. Arch Pathol Lab Med. 2007; 131: 1776-81. https://goo.gl/FFhceF
- Sun B, Qie S, Zhang S, Sun T, Zhao X, Gao S, et al. Role and mechanism of vasculogenic mimicry in gastrointestinal stromal tumors. Hum Pathol. 2008; 39: 444-51. https://goo.gl/iyL2ci
- Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000; 156: 361-81. https://goo.gl/1GvsCq
- Yue WY, Chen ZP. Does vasculogenic mimicry exist in astrocytoma? J Histochem Cytochem. 2005; 53: 997-1002. https://goo.gl/m41NEK
- Liu XM, Zhang QP, Mu YG, Zhang XH, Sai K, Pang JC, et al. Clinical significance of vasculogenic mimicry in human gliomas. J Neurooncol. 2011; 105: 173-179. https://goo.gl/QWYm55
- Chen Y, Jing Z, Luo C, Zhuang M, Xia J, Chen Z, et al. Vasculogenic mimicry potential target for glioblastoma therapy: an In vitro and in vivo study. Med Oncol. 2012; 29: 324-31. https://goo.gl/ayyfLJ
- Verkman, A.S. Physiological importance of aquaporin water channels. Ann. Med. 2002; 34: 192-200. https://goo.gl/ddaKoP
- Liu X, Wang X, Du W et al. Suppressor of fused (Sufu) represses Gli1 transcription and nuclear accumulation, inhibits glioma cell proliferation, invasion and vasculogenic mimicry, improving glioma chemo-sensitivity and prognosis. Oncotarget. 2014; 5: 11681-11694. https://goo.gl/1N1Cyh
- Du WZ, Feng Y, Wang XF, Piao XY, Cui YQ, Chen LC, et al. Curcumin suppresses malignant glioma cells growth and induces apoptosis by inhibition of SHH/GLI1 signaling pathway In vitro and vivo. CNS Neurosci Ther. 2013: 19: 926-936. https://goo.gl/kE955k
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005; 434: 786-792. https://goo.gl/J2qmbF
- Mei X, Chen YS, Chen FR, Xi SY, Chen ZP. Glioblastoma stem cell Differentiation into Endothelial cells evidenced through live-cell imaging. Neuro Oncol. 2017; 19: 1109-1118. https://goo.gl/kT4AoZ
- Sonja Klebe, Kim Griggs, Yuen Cheng, Jack Driml, Douglas W. Henderson, et al. Blockade of aquaporin 1 inhibits proliferation, motility, and metastatic potential of mesothelioma In vitro but not in an in vivo model. Dis. Markers. 2015; 286719: 9. https://goo.gl/b4Ek9J
- Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Dowell JM, Reardon DA, Quinn JA, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007; 13: 1253-9. https://goo.gl/UXmJPH
- Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009; 27: 740-5. https://goo.gl/qH8HLk
- Banerjee S, Khajanchi S, Chaudhuri S. A Mathematical Model to Elucidate Brain Tumor Abrogation by Immunotherapy with T11 Target Structure. PLoS One. 2015; 10: e0123611. https://goo.gl/UjczKB
- Khajanchi S. Uniform Persistence and Global Stability for a Brain Tumor and Immune System Interaction, Biophys. Rev. Lett. 2017; 12: 1-22. https://goo.gl/HxcLVN
- Chen YS, Chen ZP. Vasculogenic mimicry: a novel target for glioma therapy. Chin J Cancer. 2014; 33: 74-9. https://goo.gl/TZR27u


Sign up for Article Alerts