Characterization, Antimicrobial Activity of Silver Nanoparticles Biosynthesized by Acanthus ilicifolius
Noor Muzamil Mohamad1, Siti Razila Abdul Razak<2, Mowaffaq Adam Ahmed2, Laina Zarisa Muhd Kamal2, Siti Nurfatimah Mohd. Shahpudin2, Rosline Sandai3, Leslie Thian Lung Than4, Saad Sabbar Dahham5, Yasser Tabana6 and Doblin Sandai2*
1Faculty of Pharmacy, Universiti Sultan Zainal Abidin, Kampus Besut, Terengganu, Malaysia2Advanced Medical and Dental Institute, Universiti Sains Malaysia, Penang, Malaysia
3Faculty of Language and Communication, Universiti Pendidikan Sultan Idris, Perak, Malaysia
4Department of Medical Microbiology and Parasitology, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Selangor, Malaysia
5Department of Science, Rustaq College of Education, Rustaq-329, Sultanate of Oman
6Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Alberta, Canada
*Address for Correspondence: Doblin Sandai, Infectomics Cluster, SAINS@BERTAM, Advanced Medical and Dental Institute, Universiti Sains Malaysia, Penang, Malaysia, Tel: +0060-456-2386; E-mail: [email protected]
Submitted: 04 April 2019; Approved: 25 April 2019; Published: 29 April 2019
Citation this article: Mohamad NM, Abdul Razak SR, Ahmed MA, Muhd Kamal LZ, Sandai D, et al. Characterization, Antimicrobial Activity of Silver Nanoparticles Biosynthesized by Acanthus ilicifolius. American J Current App Res Microbiol. 2019;1(1): 009-016.
Copyright: © 2019 Mohamad NM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Acanthus ilicifolius; Biosynthesis; Silver nanoparticles; Antibacterial activity
Download Fulltext PDF
Background/Purpose: The reduction solution was aqueous extracted from Acanthus ilicifolius for biosynthesis of silver nanoparticles as green approach. It is less harmful and more economical as compared to physical and chemical methods.
Methods: Ratio of 1: 10 mixtures of 100 mg/ mL of aqueous extract and 5 mM of silver nitrate were incubated for 24 hours at 40°C with 150 rpm in incubator shaker. The formation of silver nanoparticles were monitored by colour changes and were characterized by UV-Vis spectrometry followed by zeta (potential) sizer analyses.
Results: The UV-Vis spectrum of biosynthesized AgNPs showed absorption at maxima 440 while zeta (potential) sizer analyses revealed the average size of biosynthesized AgNPs was 94 nm with zeta potential value of -4.08 mV for the biosynthesized AgNPs. The antibacterial activity for Staphylococcus aureus (ATCC 33591), Bacillus cereus (ATCC 14579), Escherichia coli (ATCC 33591) and Pseudomonas aeruginosa (ATCC 27853) showed an inhibition zone of 16, 15, 14 and 20 mm respectively by using well diffusion method. Minimal Inhibitory Concentration (MIC) as well as Minimal Bactericidal Concentration (MBC) of this silver nanoparticles product accessed by MTT assay was lower than 0.0843 µg/ mL. This concluded, biosynthesized AgNPs was produced and showing antimicrobial activities against selected bacteria.
Introduction
The development of biosynthesized AgNPs as antibacterial agents are necessary as alternative and complement to antibiotics to overcome bacterial resistance towards antimicrobial issues [1]. The biosynthesized AgNP properties, such as crystallographic surface structures and high surface-to-volume ratios, made it possible to be an antibacterial agent along with the other promising nanomaterials such as gold, zinc, titanium and other metals [2]. The smaller particles with a larger surface-to- volume ratio have a higher antibacterial activity as compared to bigger particles [3]. Beside the size, the antibacterial activity of AgNPs is also influenced by shape. The synthesized AgNPs using different saccharide types and other types of biomolecules and biological materials increased antimicrobial activity against bacteria, including highly multidrug-resistant strains such as methicillin-resistant Staphylococcus aureus, MRSA and Carbapenem-Resistant Enterobacteriaceae (CRE) [4-6]. The AgNPs usually interact with the Gram-negative organism such as Escherichia coli. The efficacy of the antimicrobial effects of AgNPs on Escherichia coli, Staphylococcus aureus and yeast was examined by Kim et al. [7] and showed that at low AgNP concentrations, complete growth inhibition was observed in Escherichia coli and yeast, whereas a mild effect was observed in Staphylococcus aureus. The efficacy of various antibiotics such as amoxicillin, clindamycin erythromycin, penicillin G, tetracycline, kanamycin, neomycin, gentamycin and vancomycin against different type of bacteria was synergistically increased in the presence of biosynthesized AgNPs [5,8].
Acanthus ilicifolius has been used traditionally for several human medication. It is used as an aphrodisiac, blood purifier, diuretics, asthma, diabetes, dyspepsia, hepatitis, leprosy, neuralgia, paralysis, ringworm, rheumatic, skin diseases, snakebite, abdominal pain, leucorrhoea and leukaemia [9-11]. In Ayurveda practice, this plant is currently being used to ease rheumatic problem and may also being used as antioxidant [12-14]. It is known that Acanthus ilicifolius contain bioactive compounds including alkaloids, triterpenoids, lignan, phenolic compounds, steroids, flavonoid, and terpenoids [15,16]. Studies on the biological activities of Acanthus ilicifolius revealed that this plant has anti-parasitic (Leishmania spp), osteoblastic, hepatoprotective, anti-inflammatory, antiulcer, antioxidant, anticancer, and antimicrobial activities. Kuppusamy et al. [17], biomolecules contained in Acanthus ilicifolius such as sugars, protein, alkaloids, triterpenoids, lignan, phenolic compounds, steroids, flavonoid, and terpenoids may contributed to the reduction and antioxidant processes in AgNps biosynthesis.
This study focused on biosynthesis of AgNPs by Acanthus ilicifolius and evaluation of their antimicrobial activity. Physic-chemistry characteristics of biosynthesized AgNps was also determined.
Methods
Biosynthesis of AgNPs by using Acanthus ilicifolius
Acanthus ilicifolius extract powder reconstituted with distilled water to form 100 mg/ mL solution. The AgNPs were prepared by mixing 100 mg/ mL Acanthus ilicifolius solution with 5 mM silver nitrate (AgNO3) solution. A ratio of 1:10 (one part of 100 mg/ mL plant extract with another 9 parts of 5 mM silver nitrate) was used in the mixture. In this study, several incubation periods were selected at 0, 1, 6, and 24 hours. The mixture then incubated in an incubator shaker at 40°C with agitation of 150 rpm for 24 hours with minimal light exposed into the incubator shaker. Reduction of AgNO3 to Ag+ ions was confirmed by the change of colour from clear (before mixing) to colloidal brown.
Characterization of biosynthesis AgNPs
Ultraviolet Visible (UV-VIS) Spectroscopy analysis: The formation of biosynthesized AgNPs were monitored by observing the colour changes at 0, 1, 6 and 24 hours and the sample were analysed by UV-Vis Spectroscopy. The reduction of silver ions in the solution was monitored by measuring the absorbance value of 1 mL of biosynthesized AgNPs. Silver nitrate (5 mM) solution was used as a blank. Based on the result obtain from this analysis, samples at 24 hours incubation time was selected for future characterization. Before analysis, the colloidal solution produced after 24 hours was divided into two parts: 1 part for analysis and 1 part for bioassay. The part for analysis was centrifuged at 12000x g for 15 minutes. After that, the supernatant as discarded, and the pellet was resuspended with sterile water before being dried for 48 hours at 45°C in the oven. Another part for bioassay was stored in refrigerator at 4°C prior to use.
Zeta (Potential) Sizer analysis: The colloidal solution of synthesized nanoparticles was centrifuged at 5000x g for 10 minutes and the supernatant was then discarded. The pellet then was dried at 60°C for 24 hours. The dried pallet then been used for Zeta (Potential) Sizer. The average size and zeta potential of silver nanoparticles samples dispersed in different water were characterized by using Zeta (Potential) Sizer Analyser. All analysis using Zeta (Potential) Sizer Analyzer was conducted at School of Materials and Mineral Resources Engineering, Engineering Campus of Universiti Sains Malaysia.
Antibacterial activity of biosynthesized AgNPs
Agar well diffusion method: The antibacterial activity of the biosynthesized AgNPs was determined by agar well diffusion method. 24-hours cultures grown on MHA medium were used to check the antibacterial activity of the tested. Three to four colonies of each bacteria strain was inoculated in 10 mL of MHB medium in culture tubes. The broth inoculums of bacteria were then adjusted to 0.5 McFarland standards. Using sterile cotton swab, the bacteria suspensions were then then inoculated by spreading a volume of the microbial inoculum over the entire agar surface. The wells then were made on the agar plate using 6 mm cork borer. Different solution of the plant extract and synthesized nanoparticles were prepared and added to respective wells. Vancomycin 30 µg disc and Gentamycin 120 µg disc were used to respective Gram positive and Gram negative while sterile water was used as negative control. The effect of synthesized silver nanoparticles and plant extract against the bacteria strains were evaluated and compared with the control used in the study. The plates were then incubated at 37 ± 2°C for 24 hours. The antibacterial activity was calculated by measuring the zone of inhibition by using standard scale.
Antimicrobial Activity Determination by MTT Method: Staphylococcus aureus (ATCC 33591), Bacillus cereus (ATCC 14579), Escherichia coli (ATCC 33591) and Pseudomonas aeruginosa (ATCC 27853) were cultured in Mueller-Hinton agar for approximately 24 hours. Colonies of those bacteria were then being used to prepare separate bacterial suspension for the determination of antimicrobial activity. Bacterial suspension briefly containing 0.5 McFarland (approximately 1.5 × 108 colony forming units/ mL) of organisms in MHB was added to various concentrations of Acanthus ilicifolius-AgNPs aqueous extract ranging from 0.0039060 to 2.0000000 mg/ ml; and Penicilin-Streptomycin solution ranging from 78.125 – 78.125 µg/ mL 10000 µg/ mL in 96-wells plates. Dilution of compound was done by double dilution manner. The plates were incubated at 37°C for 18-24 hours. After incubation, fifteen µl of [3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide], MTT solution (5 mg/ mL) was added to each single well and the 96-well micro-titer plates were incubated for 60 minutes at 37°C. After 60 minutes incubation with MTT solution, 100 μL of the MTT solution was carefully removed and discarded and being replaced with 100 μL dimethyl sulfoxide. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) were determined by the MTT Assay. The MIC concentration of tested compound is defined as the lowest concentration visible purple blue color whereas MBC concentration of tested compounds of bacteria after overnight incubation of cultures at. The MBC value is the lowest concentration of compounds tested which do not change the color of MTT to purple blue. MBC is the lowest concentration of a tested compound that results in killing 99.9% of the bacteria being tested [18-20].
Results
Herbarium voucher specimen identification
The plant material was identified as Acanthus ilicifolius Wallich, N from a family of Acanthaceae. A copy of the herbarium voucher specimen was numbered as USM Herbarium 11756 at Universiti Sains Malaysia. This plant is a kind perennial herb plant that is widely found in mangrove swamps and marshes near sea level. This plant is an erect herb, and may grow up to 2.5 m tall, with spiny, often yellowish stem. All the leaves like those holly with leaf blade dark green, slightly too deeply lobed with spine at each tip. The inflorescence of the plant are large and showy petal those neatly organized -spike at branch tips. In addition, the light violet capsules squares and slightly flattened and will explode when ripe.
Biosynthesized AgNPs
The formation of Acanthus ilicifolius-AgNPs can be observed according to the colour change in the mixture. Upon mixing of 100 mg/ mL of Acanthus ilicifolius aqueous extract with 5 mM silver nitrate solution in 1:10 ratio, the solution turned to pale yellow from colourless solution. The solution change to yellowish brown within 1 hour. The colour continued to become darker as further being incubated. Figure 1 shows the colour change observed in mixture of Acanthus ilicifolius aqueous extract and 5 mM of silver nitrate after 6 hours of reaction and the colour remain after 24 hours visually.
UV-Vis spectroscopy analysis
Figure 2 shows the UV-Vis spectra recorded from Acanthus ilicifolius aqueous extract reaction with silver nitrate solution at different time intervals. The spectrum showed maximum absorption peaks around 440 nm.
Zeta (Potential) Sizer analysis
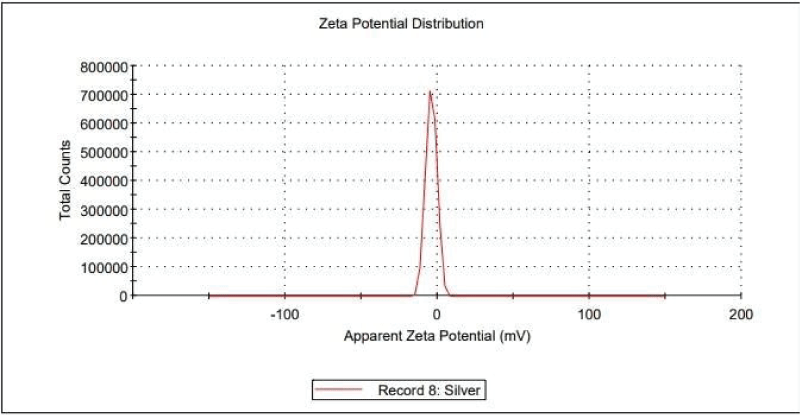
The stability of Acanthus ilicifolius-AgNPs in solution was determined by using zeta potential analysis in measuring electrical charges on the surface of Acanthus ilicifolius-AgNPs. As shown in figure 3 and table 1, the zeta potential value of Acanthus ilicifolius-AgNPs was – 4.08 mV with a peak area of 100% intensity. The potential of synthesized silver nanoparticles is specified in water as dispersant. Particles obtained are polydisperse. Mixed with sizes within 10 to 400 nm, the average diameter of the particles were obtained 94 nm by zeta sizer analysis.
Antimicrobial activity of biosynthesized AgNPs
The antimicrobial activity of silver nanoparticles synthesized by plant extracts has been studied against various pathogenic organisms such as Staphylococcus aureus, Bacillus cereus, Escherichia coli and Pseudomonas aeruginosa by well- diffusion method. The diameter of the zones of inhibition (mm) around each was synthesized with silver nanoparticle, Acanthus ilicifolius aqueous, Vancomycin 30 µg disc and Gentamycin 120 µg disc shown in table 2.
For the analysis of MTT results showed no penicillin: streptomycin (10000 unit: 10000 μg/ mL solution) concentrations had an effect on S. aureus as well as in the plant extract. While Acanthus ilicifolius-AgNPs inhibited completely tested bacteria (Staphylococcus aureus, Bacillus cereus, Escherichia coli and Pseudomonas aeruginosa) were found to be 0.0843 µg/ mL. MIC and MBC value of biosynthesized AgNPs, Acanthus ilicifolius of aqueous extract and penicillin-streptomycin solution on tested bacterial is shown in table 3.
Discussions
Green biological method of AgNPs biosynthesis is the chosen method as wide variety of plant can be exploited for this purpose. Apart for being abundantly found anywhere and cheap source, the plants contain a lot of active metabolites and safer as compared to hazardous chemicals in producing AgNPs. In addition, utilizing plants to produce AgNPs is a simple procedure and able to produce a rapid and high yield of AgNPs as plants possess higher reducing capabilities on silver ion.
Acanthus ilicifolius
We use Acanthus ilicifolius Wallich, N, a plant that is usually found in mangrove swamp to syntheses the AgNPs. This plant was collected from Northern West Malaysia. According to the villagers, the plant has prevented the infection of the poultry from Newcastle Disease Virus when the epidemic spread. The plant has also been utilized for dyspepsia, paralysis, asthma, headache, and rheumatism, antidote for snakebite and skin diseases traditionally [12]. In addition to medicinal properties, this plant may serve as bio- indicator of contamination to the swampy area Venkatesalu et al. [21] as this plant may accumulates heavy metals such as copper, cadmium, zinc and mercury into their system and the heavy metals content could be analyzed in the laboratory to indicate the severity of contamination in the area. Apart form that the property of bioaccumulation, it is also potential to be employed for bioremediation of the contaminated area [21-24]. Acanthus ilicifolius is sometimes misidentified as Acanthus ebracteatus, a plant growing in swamps like Acanthus ilicifolius because of its almost identical appearance. Both Acanthus ilicifolius and Acanthus ebracteatus are not clearly distinguished from each other [25]. Acanthus ilicifolius and Acanthus ebracteatus differ due to the presence and absence of bracteoles, the flower colour and the position of inflorescences and direction of the axial spines of the stem [26]. Acanthus ilicifolius was suggested to contain phenols, flavonoids, alkaloids, terpenoids, sterols, tannins, glycosides, saponins, quinones, coumarins and several other metabolites including sugars, proteins and fatty acid [14,16].Those biomolecules in the plant may be suggested to facilitate the biosynthesis of the AgNPs.
Biosynthesized AgNPs
According to previous studies done by other researchers, some use fresh leaves to prepare the plant extract for getting silver nanoparticles biosynthesis while others preferred to use the extract prepared using dried powder or minced dried plant directly for synthesizing nanoparticles or studying the bioactive compound of the plant or bioactivity. Ahmed et al. [27] produced AgNPs using aqueous extract of Azadiracta indica. The aqueous extract was prepared by boiling the chopped dried leaves in water. While another study done by Radhiyatul et al. [28], aqueous extract of Vernonia cinera was prepared by boiling the dried ground plant coarse powder in water and the extract was utilised to produce AgNPs. Musa et al. [29], employed the same methods of AgNps syntheses with Radhiyatul et al. by replacing plant with mushroom Pleurotus sajor- caju. In this study, we prepare the plant extract by using the dried powder of raw plant then the extract was subjected to freeze drying and maceration to fine powder by maceration in mortar and pestle (with addition of liquid nitrogen to facilitate the maceration). We suggested this method of using the final fine powder as compared to previously done [28-30]. With this method, the plant extract preparation was properly prepared with exact amount and content being used. This maybe one component of the plant or herbal standardization. According to Bezerra et al. [31], standardized extracts consisting of high quality plant-based preparations with relatively constant reference compounds and stringent quality controls throughout the production process. Standardization is not only ensure the quality, effectiveness and reliability of phytomedicine in uniform and stable active ingredient, but also improve the yield during the extraction process. Reduction of Ag+ in the silver nitrate solution during the reaction with the compound present in Acanthus ilicifolius aqueous extract was observed by colour change from pale yellow immediately after mixing and to yellowish brown after 1 hour. When the mixture further incubated for 2 hours and up to 24 hours the colour solution become darker (dark brown-greyish) due to silver ions reduction indicating the formation of AgNPs [32]. Similar colour change was observed using other plant extract [33-35]. The characteristic of dark brown colour of AgNPs provided an easier visual signature indicating the formation of biosynthesized AgNPs.
UV-Vis spectroscopy and zeta (potential) sizer
the examples methods used to confirm or verify the formation of biosynthesized AgNPs. UV-Vis Spectrometry has been utilized widely to characterize electronic structure and optical properties of the forming AgNPs. Surface Plasma Resonance (SPR) occurs when charged nanoparticles oscillate in electron clouds to absorb electromagnetic radiation released during the process at certain frequencies recorded as magnetic wavelength in the UV-Vis, and it is heavily influenced by the size and shape of the particles. For example, spherical particles are known to indicate peak in the wavelength range of 415-490 nm [29]. UV-Vis spectra recorded from the plant extract showed maximum peak within 440 nm after 24 hours incubation could be considered as spherical particles as mention earlier. The Zeta potential measurements of silver nanoparticles synthesized was – 4.08 mV with a peak area of 100% intensity. While Zeta sizer was used to analyse the diameters of the biosynthesized AgNPs. The size mix within 10 to 400 nm with the average of 94 nm. The zeta potential measurements of biosynthesized AgNPs, which revealed negative charge of biosynthesized AgNPs was completely instable and tend to rapid coagulation or flocculation of the nanoparticles produced. The higher value of either negative or positive zeta potential, AgNPs is more stable and have the stronger the prevention of biosynthesized AgNPs from its aggregation. The stability of biosynthesized AgNPs colloid with zeta potential value less than 5 is tend to coagulate and flocculate rapidly while the values ranging from 10-30 is incipiently instable. AgNPs colloid which having the zeta potential value greater than 40 may be considered stable [36,32].The Zeta potential measures the electrostatic potential of the double electric layers that surround the nanoparticles in a solution [29].
Antimicrobial activity of Acanthus ilicifolius-AgNPs
Staphylococcus aureus and Bacillus cereus were used as representative of Gram negative bacteria while Pseudomonas aeruginosa and Escherichia coli were used as representative for Gram-negative bacteria. Apart from this, those bacteria were chosen as they are the commonly pathogen found in skin and in gastrointestinal tract. Current study was done by well diffusion method, showed that both biosynthesized AgNPs and 2 mg of Acanthus ilicifolius extract solution inhibited growth of all bacteria strains tested which were Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Bacillus cereus. It was found that Gram-positive bacteria, which were Staphylococcus aureus and Bacillus cereus was more effective than standard antibiotic (vancomycin) used as their zone of inhibition larger than vancomycin 30 µg disc. While for Gram- negative bacteria, the zone of inhibition of biosynthesized AgNPs was 14 mm for Escherichia coli, and 20 mm for Pseudomonas aeruginosa whereas 18 mm and 20 mm respectively for 2 mg Acanthus ilicifolius extract. The finding showed smaller zone of inhibition as compared to Gentamycin antibiotic disc 120 µg (27 mm for Pseudomonas aeruginosa and 25 mm for Escherichia coli).
The inhibition of bacteria by biosynthesized AgNPs is comparable with previously done recently by other researchers. El-Naggar et al. [37] reported the their AgNPs synthesized by using intracellular protein of cyanobacteria showed 16 mm and 21 mm of zone of inhibition on Staphylococcus aureus and Escherichia coli respectively. The finding was parallel with the current study on Staphylococcus aureus but contrarily they obtained larger zone of inhibition on Escherichia coli. Karimipour S.N. and Tanomand A [38] tested the commercially available AgNPs on Pseudomonas aeruginosa and reported that AgNPs inhibited the growth of Pseudomonas aeruginosa by well diffusion method with the zone of inhibition ranging from 10-15 mm depending on concentration and the particle size of commercial AgNPs used. Biosynthesized AgNPs was suggested to be having slightly better antibacterial activity compare to commercial biosynthesized AgNPs against Pseudomonas aeruginosa.
Biosynthesized AgNPs was found to have antibacterial activity against Staphylococcus aureus by having 16 mm zone of inhibition comparable to standard antibiotic disc of vancomycin used in this study with 15 mm of inhibition zone. Other study was done by El-Naggar et al. [37] using biosynthesized AgNPs as mentioned earlier obtained similar zone of inhibition with current study while Buszewski et al. obtained smaller zone of inhibition against Staphylococcus aureus. They also claimed the zone of inhibition by well diffusion method increased when AgNPs combined with gentamycin against Staphylococcus aureus as well as Bacillus subtilis, Klebsiella pneumoniae and Pseudomonas aeruginosa tested. Aqueous extract of Acanthus ilicifolius also found to have antibacterial activity on tested bacteria. The inhibition zone obtained by using this extract against all tested bacteria were comparable to AgNPs as well as standard antibiotic disc used. Contrary, the aqueous extract was found to have greater zone of inhibition which was 18 mm against Escherichia coli than AgNPs (14 mm). We suggest this finding shall be repeated and validated in the future as this maybe a questionable finding obtained. We also suggest to further study on the potential of this aqueous extract and possible resistance trait arising if the similar pattern of finding obtain in the future against Escherichia coli.
Interestingly, we found our synthesised nanoparticles inhibit methicillin resistant Staphylococcus aureus (MRSA) strain (ATCC 33591) used in this study. Staphylococcus aureus is a Gram-positive bacterium. This coagulase producing bacteria that is commonly regarded as a normal flora or commensal of the skin and human nasal cavity and various animal species. MRSA is one of the major risk pathogenic bacteria associated with the antimicrobial agent resistance evolution [39]. Most MRSA strain produce penicillinase enzyme (β-lactamase) that it is main cause of resistance to antibiotics as well as other enzyme produced by their counterparts [40]. While these resistant bacteria cause wide range of infections with difficult to be inhibited by ordinary drugs, we may employ this green synthesized silver nanoparticles in order to combat those resistant microorganisms.
Evaluation antimicrobial activity of Acanthus ilicifolius-AgNPs and Acanthus ilicifolius aqueous extract were also been done by MTT Assay in addition to well diffusion method. In this study, MTT method was choose over other methods such as broth micro dilution and colourimetric assay (resazurin). Grela et al. [41] in their review described currently the MTT assay become more popular due to its advantages. This test is rapid, executed properly and accurate compared to other methods. A lot of sample maybe placed in the microplate and maybe considered cost effective compared to other method mention previously. So far, the MTT assay offers possibility of replacing conventional standard colony counts of MIC/MBC to modified MTT based MIC/MBC evaluation in determination of bacteria susceptibility and optimal doses of drug or natural compound to inhibit microorganism. The absorbance value obtained under optimum condition is proportionally with the count of living cell [42].
Conclusion
Our results indicated that Acanthus ilicifolius extracted can be used for efficient synthesis of AgNPs using inexpensive, eco-friendly and non-toxic method. Furthermore, the obtained biosynthesized AgNPs exhibited unique physicochemical and biochemical properties. Our finding provides insight into the development of new antimicrobial agents with enhancement of the antibacterial mechanism against clinical bacteria.
Acknowledgments
The study was supported by the Universiti Sains Malaysia USM RUI grant; 1001/CIPPT/812196.
- Le Ouay B, Stellacci F. Antibacterial activity of silver nanoparticles: as surface science insight. Nano Today. 2015; 10: 339-354. https://bit.ly/2XLJ96a
- Abou El-Nour KMM, Eftaiha A, Al-Warthan A, Ammar RAA. Synthesis and applications of silver nanoparticles. Arabian Journal of Chemistry. 2010; 3: 135-140. https://bit.ly/2INVOT5
- Baker C, Pradhan A, Pakstis L, Pochan DJ, Shah SI. Synthesis and antibacterial properties of silver nanoparticles. J Nanosci Nanotechnol. 2005; 5: 244-249. https://bit.ly/2DBmiCK
- Ali SG, Ansari MA, Khan HM, Jalal M, Mahdi AA, Cameotra SS. Antibacterial and Antibiofilm potential of green synthesized silver nanoparticles against imipenem resistant clinical isolates of P. aeruginosa. BioNanoScience. 2018; 8: 544-553. https://bit.ly/2ZwSFw1
- Buszewski B, Railean-Plugaru V, Pomastowski P, Rafińska K, Szultka-Mlynska M, Golinska P, et al. Antimicrobial activity of biosilver nanoparticles produced by a novel Streptacidiphilus durhamensis strain. J Microbiol Immunol Infect. 2018; 51: 45-54. https://bit.ly/2GGvhDo
- Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007; 73: 1712-1720. https://bit.ly/2ITfWCp
- Kim JS, Kuk E, yu kn, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007; 3: 95-101. https://bit.ly/2GHcmJR
- Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine. 2007; 3: 168-171. https://bit.ly/2XJZUyO
- Juffe Bignoli D. Acanthus ilicifolius (Holy Mangrove). 2013; 8235.
- Ramanathan T, Saranya A, Kesavanarayanan KS, Adam A. Traditional medicinal uses, chemical constituents and biological activities of a mangrove plant, Acanthus ilicifolius Linn. : A Brief Review. American-Eurasian J Agric & Environ Sci. 2015; 15: 243-250. https://bit.ly/2ZxIbMF
- Xie LS, Liao YK, Huang QF, Huang MC. Pharmacognostic studies on mangrove Acanthus ilicifolius. Zhongguo Zhong Yao Za Zhi. 2005; 30: 1501-1503. https://bit.ly/2GHfRzZ
- Singh D, Aeri V. Phytochemical and pharmacological potential of Acanthus ilicifolius. J Pharm Bioallied Sci. 2013; 5: 17-20. https://bit.ly/2GGBhgE
- Bandaranayake WM. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetlands Ecology and Management. 2002; 10: 421-452. https://bit.ly/2GueZxi
- Paul T, Ramasubbu S. The antioxidant, anticancer and anticoagulant activities of Acanthus ilicifolius L. roots and Lumnitzera racemosa Willd. Leaves, from southeast coast of India. Journal of Applied Pharmaceutical Science. 2017; 7: 081-087. https://bit.ly/2XIdxhT
- Firdaus M, Prihanto AA, Nurdiani R. Antioxidant and cytotoxic activity of Acanthus ilicifolius flower. Asian Pac J Trop Biomed. 2013; 3: 17-21. https://bit.ly/2J313xv
- Wöstmann R, Liebezeit G. Chemical composition of the mangrove hollyAcanthus ilicifolius (Acanthaceae) - review and additional data. Senckenbergiana Maritima. 2008; 38: 31-37. https://bit.ly/2II2Vft
- Kuppusamy P, Yusoff MM, Maniam GP, Govindan N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications- An updated report. Saudi Pharmaceutical Journal. 2016; 24: 473-484. https://bit.ly/2XKl680
- Chuah EL, Zakaria ZA, Suhaili Z, Abu Bakar S, Mohd Desa MN. Antimicrobial activities of plant extracts against Methicillin-Susceptible and Methicillin-Resistant. Journal of Microbiology Research. 2014; 4: 6-13. https://bit.ly/2Dze3ah
- Misra R, Sahoo SK. Antibacterial activity of doxycycline-loaded nanoparticles. Methods Enzymol. 2012; 509: 61-85. https://bit.ly/2PDUanD
- Salem W, Schild S, Schneditz G, Dornisch E. In vitro effects on biofilm viability, cytotoxicity and antibacterial activities of green Ag and ZnO nanoparticles against Nontypeable Haemophilus influenzae strains. Journal of Experimental and Applied Animal Sciences. 2015; 1: 369-383. https://bit.ly/2UAVBnr
- Agoramoorthy G, Chen FA, Venkatesalu V, Shea PC. Bioconcentration of heavy metals in selected medicinal plants of India. J Environ Biol. 2009; 30: 175-178. https://bit.ly/2L7S3JY
- Shackira AM, Puthur JT. Enhanced phytostabilization of cadmium by a halophyte-Acanthus ilicifolius L. Int J Phytoremediation. 2017; 19: 319-326. https://bit.ly/2GIiwcU
- Shackira AM, Puthur JT, Nabeesa Salim E. Acanthus ilicifolius L. a promising candidate for phytostabilization of zinc. Environ Monit Assess. 2017; 189: 282. https://bit.ly/2XNXMpP
- Wahwakhi S, Kusmana C, Iswantini D. Potency of Acanthus ilicifolius as phytoremediation agent against copper pollution in Jagir River estuary, Wonorejo Village, Surabaya, Indonesia. AACL Bioflux. 2017; 10: 1186-1197. https://bit.ly/2UUIdjb
- Kathiresan K, Rajendran N. Mangrove ecosystems of the Indian Ocean region. Indian Journal of Marine Sciences. 2005; 34: 104-113. https://bit.ly/2L1s5b3
- Ragavan P, Saxena A, Mohan PM, Jayaraj RSC, Ravichandran K. Taxonomy and distribution of species of the genus Acanthus (Acanthaceae) in mangroves of the Andaman and Nicobar Islands, India. Biodiversitas. 2015; 16: 225-237. https://bit.ly/2DBnsOE
- Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. Journal of Advanced Research. 2016; 7: 17-28. https://bit.ly/2ZA6gCM
- Radhiyatul A, Zani M, Ahmad H, Razila S, Razak A. Synthesis and characterisation of silver nanoparticles using vernonia cinerea aqueous extract and their cytotoxicity activity against Kasumi-1 Cell Line. Jurnal Teknologi. 2016; 78: 57-65. https://bit.ly/2Pw0z4b
- Musa SF, Yeat TS, Kamal LZM, Tabana YM, Ahmed MA, El Ouweini A, et al. Pleurotus sajor-caju can be used to synthesize silver nanoparticles with antifungal activity against Candida albicans. J Sci Food Agric. 2018; 98: 1197-1207. https://bit.ly/2ZA57em
- Ahmed S, Saifullah, Ahmad M, Swami BL, Ikram S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. Journal of Radiation Research and Applied Sciences.2016; 9: 1-7. https://bit.ly/2XIRtUj
- Bezerra JDP, Sandoval-Denis M, Paiva LM, Silva GA, Groenewald JZ, Souza-Motta CM, et al. New endophytic Toxicocladosporium species from cacti in Brazil, and description of Neocladosporium gen. nov. IMA Fungus. 2017; 8: 77-97. https://bit.ly/2L6K4Np
- Wypij M, Czarnecka J, Świecimska M, Dahm H, Rai M, Golinska P. Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from Streptomyces xinghaiensis OF1 strain. World J Microbiol Biotechnol. 2018; 34: 23. https://bit.ly/2UJhYad
- Baghayeri M, Mahdavi B, Hosseinpor-Mohsen Abadi Z, Farhadi S. Green synthesis of silver nanoparticles using water extract of Salvia leriifolia: Antibacterial studies and applications as catalysts in the electrochemical detection of nitrite. Applied Organometallic Chemistry. 2018; 32: e4057. https://bit.ly/2GB5xs6
- Kumar M, Bansal K, Gondil VS, Sharma S, Jain DVS, Chhibber S, et al. Synthesis, characterization, mechanistic studies and antimicrobial efficacy of biomolecule capped and pH modulated silver nanoparticles. Journal of Molecular Liquids 2018; 249: 145-1150. https://bit.ly/2XLgpuA
- Perugu S, Nagati V, Bhanoori M. Green synthesis of silver nanoparticles using leaf extract of medicinally potent plant Saraca indica: a novel study. Applied Nanoscience. 2016; 6: 747-753. https://bit.ly/2GJ4gk9
- Devi Raveena R, Premalatha R, Saranya A. Comparative analysis of phytochemical constituents and antibacterial activity of leaf, seed and root extract of Cajanus cajan (L.) Mill sp. International Journal of Current Microbiology and Applied Sciences. 2016; 5: 485-494. https://bit.ly/2GBba9H
- El-Naggar NE, Hussein MH, El-Sawah AA. Phycobiliprotein-mediated synthesis of biogenic silver nanoparticles, characterization, in vitro and in vivo assessment of anticancer activities. Sci Rep. 2018; 8: 8925. https://bit.ly/2vnVZM8
- Karimipour SN, Tanomand A. Evaluating the antibacterial activity of the nanoparticles of silver on Pseudomonas aeruginosa. International Journal of Medical Research & Health Sciences. 2016; 5: 424-430. https://bit.ly/2PE2t2N
- Suhaili Z, Rafee P, Mat Azis N, Yeo CC, Nordin SA, Abdul Rahim AR, et al. Characterization of resistance to selected antibiotics and Panton-Valentine leukocidin-positive Staphylococcus aureus in a healthy student population at a Malaysian University. Germs. 2018; 8: 21-30. https://bit.ly/2L3lVqT
- Moridikia A, Zargan J, Sobati H, Goodarzi HR, Hajinourmohamadi A. Anticancer and antibacterial effects of Iranian viper (Vipera latifii) venom; an in-vitro study. J Cell Physiol. 2018; 233: 6790-6797. https://bit.ly/2XO7Yi5
- Chuah EL, Zakaria ZA, Suhaili Z, Abu Bakar S, Mohd Desa MN. Antimicrobial activities of plant extracts against methicillin-susceptible and methicillin-resistant. Journal of Microbiology Research. 2014; 4: 6-13. https://bit.ly/2Dze3ah
- Grela E, Kozłowska J, Grabowiecka A. Current methodology of MTT assay in bacteria - A review. Acta Histochem. 2018; 120: 303-311. https://bit.ly/2Pw2CVV




Sign up for Article Alerts