Study of Etiological Profile and Resistance Pattern of Spontaneous Bacterial Peritonitis in Chronic Liver Disease
Showkat A Kadla, Mohamad Muzzafer Mir*, Majid Khalil Rather, Nisar A Shah and Zeeshan A Wani
Department of Gastroenterology and Hepatology, Government Medical College, Srinagar, Jammu Kashmir
*Address for Correspondence: Mohamad Muzzafer Mir, Senior resident, Department of Gastroenterology And Hepatology, Government Medical College, Srinagar, Jammu Kashmir, India, Tel: +91-941-908-7666; E-mail: [email protected]
Submitted: 16 July 2019; Approved: 25 October 2019; Published: 02 November 2019
Citation this article: Kadla SA, Mir MM, Rather MK, Shah NA, Wani ZA. Study of Etiological Profile and Resistance Pattern of Spontaneous Bacterial Peritonitis in Chronic Liver Disease Int J Hepatol Gastroenterol. 2019; 5(1): 012-015.
Copyright: © 2019 Kadla SA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Spontaneous bacterial peritonitis; Community acquired; Hospital acquired; Hospital care associated; Multi drug resistant; Culture
Download Fulltext PDF
Introduction: Landscape of etiological profile and microbiological resistance of Spontaneous Bacterial Peritonitis (SBP) in Chronic Liver Disease (CLD) is continuously changing. Early antibiotic treatment of SBP is crucial but spread of Multidrug Resistant (MDR) organism makes its current management challenging. Our study provides fresh insight into its etiology and resistance profile to design better empiric regimen.
Objective: Study etiological profile and resistance pattern of SBP in CLD
Methods: This prospective observational study was conducted at Government Medical College, Srinagar from April 2018 to March 2019.
Results: Prevalence of SBP at our center was 38.09%. Eighty two patients were included in study. Mean age of cases was 59.09 ± 12.90 years. Culture Positive SBP (CP-SBP) were 45 (54.87%), only 1 (2.22%) was fungal. Gram Negative Bacteria (GNB) were in 68.19% of bacterial cultures. Escheria coli was cultured in 25 (55.55%) bacterial cultures of which 12 (48%) were MDR and 1 Extended Drug Resistance (XDR); Staphylococcus aureus in 13 (28.88%) of which 3 (23%) were MDR. Among CP-SBP, Community Acquired-SBP (CA-SBP) were 26 (57.8%). Drug resistance in CA-SBP was high 34% and in hospital acquired SBP was 88%. Resistance to Quinolones and third generation Cephalosporins was also high ranging from 60% to 70%.
Conclusion: In our study prevalence of SBP was 38.09%. Infections caused by GNB were 67% and antibiotic resistance approaching 60%. Resistance was highest to current first line therapy. We suggest upgrade in empirical therapy for SBP according to evolving profile of organism and keeping in view risk factors for MDR and patient condition for better outcome.
Introduction
Spontaneous Bacterial Peritonitis (SBP) is an infection of the previously sterile Ascitic Fluid (AF), without any apparent intra-abdominal source of infection in patients of Chronic Liver Disease (CLD) [1]. It was first described by Conn and Fessel in patients with hepatic cirrhosis in 1906-1907 [2]. The prevalence of SBP varies from 1.5-3.5% in out-patients and 10-30% in hospitalized patients [3,4]. Factors associated with SBP include age, history of SBP [4], gastrointestinal bleeding [4,5]. Severity of liver dysfunction scores including the Child Turcotte Pugh (CTP) score or Model for End-Stage Liver Disease (MELD) score, neutrophil count, low protein concentration (< 1.5 g/ dL) in the ascitic fluid, and long term Proton Pump Inhibitors (PPIs) use has been reported as a predictive factor [6-11] for SBP. In hospital mortality for first episode of SBP is 10-50% depending on various risk factors [12,13]. Recurrence rates are high, more than 70% within one year [14,15].
In cirrhosis disturbance in microcirculation of intestinal mucosa, results in a reduction of mucosal blood flow, intestinal bacterial overgrowth, impaired mucosal integrity [16-18] and deficiencies in local host immune defences are possible mechanisms for bacterial translocation [19,20]. Catheters and other equipment used during invasive procedures represent other possible sources of infection.
The gold standard for diagnosis of SBP consists of count ≥ 250 cells/ mm³ and/or a positive AF culture without any evidence of intra-abdominal infectious source. Culture negative SBP (CN-SBP) is defined as negative ascitic fluid culture with neutrophil count of ≥ 250 cells/ mm³ in ascitic fluid [21]. Culture positive SBP (CP-SBP) is seen in 35%-65% of SBP patients [22-26]. Enteric bacteria are the most common etiological agent [17]. Frequency of Multi Drug Resistance (MDR) bacteria in Hospital Acquired SBP (HA-SBP) is 20%-35% [11,27] and 4%-16% in Community-Acquired SBP (CA-SBP) [28]. Third-generation cephalosporins have been the most frequently used antibiotics in the treatment of SBP since 1985 [29]. They were effective for CA-SBP in CLD, with resolution rates of around 80% in past [30], but the development of resistance to third-generation cephalosporins is of great concern. Resistance can result in failure to respond to initial empirical therapy with a third-generation cephalosporin in 33%-75% of cases [19,25]. Recent studies indicate that third-generation cephalosporins are not appropriate for the treatment of hospital acquired infections in patients with CLD [31] because of effectiveness as low as 40% related to an increase in the prevalence of MDR bacteria in nosocomial infections [9,26], that is why SBP treatment recommendations distinguish between CA-SBP and HA-SBP [9,32]. Moreover resistance to third-generation cephalosporins occurred in 7.1% of CA-SBP, 21.1% of health care associated SBP (HCA-SBP) and 40.9% of Hospital Acquired SBP (HA-SBP) in recent past [33]. Some studies recommend combination regimen of meropenem and daptomycin for the management of HA-SBP [25,26], and some recommend cefoperazone/sulbactam or piperacillin/tazobactam for the empirical treatment of SBP [34].
Overall paradigm of SBP is constantly changing with easy access to hospitals and ever increasing use of antibiotics. Early antibiotic treatment of SBP is crucial. With spread of MDR organisms its current management is still challenging. There is a constant need to evaluate this infection, by observing its behaviour, new strategies aiming towards diagnostic improvement and management can be sought. Our study provides fresh insight into its etiology and resistance profile to design better empiric regimen.
Objective
Study etiological profile and resistance pattern of SBP in CLD.
Materials and Methods
This prospective observational study was conducted in department of gastroenterology and hepatology, superspeciality hospital, Shreenbagh, Srinagar. It is a twenty seven bedded department with round the clock gastroenterology services.
All patients of cirrhosis and ascites with possibility of SBP more than 10 years of age were recruited from out-patient Department of Gastroenterology and Hepatology, and medical emergency of government medical college; Srinagar over one year period. A predesigned structured proforma was used to record patient’s demographics, clinical presentation and laboratory results.
Exclusions
Etiology of ascitis other than liver disease, recent antibiotics use (within two weeks), suspected or conformed intra-abdominal source of infection like surgery or trauma, children under 10 years of age and those who did not consented to participate.
Paracentesis (only diagnostic tap) was performed bedside with under mentioned protocol:
1. Performed using standard aseptic precaution for all study participants.
2. Twenty milliliter syringes with 20 G (gauge) needle used for ascitic fluid (AF) tap in left iliac fossa or midline below umbilicus at bedside.
3. Total 20 milliliters AF was collected from each patient.
4. Ten ml for AF detailed biochemical and cytological report.
5. Ten ml of AF inoculated in blood culture bottles at the bed side using aseptic technique and send for microbiology (for aerobic and anaerobic culture).
6. Blood sample (10 ml) was collected at same time to perform serum/plasma based blood work up as deemed necessary.
Severity of liver disease was assessed by:
Child Turcotte Pugh (CTP) score: Depending of sum of these five variables patients are divided into three classes; A (score of 5-6), B (score of 7-9) and C (score of 10-15). Class A has 1 year survival of 100% and 2 year survival of 90%. Class B has 1 year survival of 81% and 2 year survival of 57%. Class C has 1 year survival of 45 % and 2 year survival of 35%.
Model of End-Stage Liver Disease (MELD) scores: Is calculated by using formula:
MELD = 9.57 X LOGe (creatinine) + 3.78 X LOGe (total bilirubin) + 11.2 X LOGe (INR) + 6.43
Originally was designed for post Transjugular intrahepatic portosystemic shunt survival. It also predicts severity of liver disease and cirrhosis related mortality (3 months). Mortality by MELD score 8% with score of 10-19, 24% with score of 20-29, 60% with score of 30-39, 81% with score of 40 and above. It is also used in allocation of organs for transplant.
Infections diagnosed on admission or within 2 days after admission were classified as Hospital Care Associated (HCA) in patients with a prior contact with the healthcare environment (hospitalization or short-term-admission for at least 2 days in the previous 90 days, residence in a nursing home or a long-term care facility or chronic hemodialysis). The infection was considered CA when present at time of admission or developed within the first 2 days after hospitalization with no history as mentioned above in HCA and HA when the diagnosis was made thereafter [32,35].
MDR was defined as non-susceptibility to at least one agent in 3 or more antimicrobial categories. Extended Drug Resistant (XDR) was defined as non-susceptibility to at least one agent in all but 2 or fewer antimicrobial categories and Pan Drug-Resistant (PDR) as non-susceptibility to all currently available agents [36].
Data analysis
Statistical analysis was conducted using SPSS ver. 16.0 for Windows (SPSS, Chicago, IL). Categorical variables were compared using the chi-square or Fisher’s exact test where appropriate. Continuous data were compared using the t-test or the Mann-Whitney test, the Kruskal-Wallis test was used for multiple comparisons, when appropriate. Quantitative variables with a normal distribution were expressed as mean values ± standard deviation and those with a non-normal distribution as median values (range). Significance level was two-sided and set to less than 0.05.
Informed consent was obtained from all participants or their attendants.
This study was cleared by institution’s review board.
Results
Prevalence of SBP in CLD presenting at our center was 38.09% (112/294). Eighty two patients were included in study. Mean age of patients was 59.09 ± 12.90 years with minimum of 20 and maximum of 89. Male were 47 (57.3%) and females were 35 (42.7%). Most common clinical presentations were ascitis 100% and hepatic encephalopathy 89%.
Majority of patients were CTP-C class i.e 73.2%. Other 15.9% and 11% were CTP-B class and CTP-A class respectively in our study.
Laboratory parameters are given in table 1. Ascitic fluid analysis is given in table 2. Mean leukocytes and neutrophils in ascitic fluid were 673 ± 340.11 and 517.95 ± 300.21 respectively. Mean SAAG was 1.30 ± 0.27.
In our study CA-SBP was present in 55 (67%), HCA-SBP in 29.3% and HA-SBP in 3.7% respectively. We found CP-SBP in 54.87% and CN-SBP in 45.13% cases. On comparing culture positivity with SBP profile, 58% of CA-SBP, 67% of HCA-SBP and 100% of HA-SBP were CP (p-value = 0.044).
We compared SBP profile with resistance pattern of pathogens. In CA-SBP, Drug Sensitive (DS) were 18(69%), MDR were 7(27%) and XDR were 1(4%). In HCA-SBP, DS were 4(25%), MDR were 8 (50%) and XDR were 4(25%). In HA-SBP, DS were 1(33%) and MDR were 2(67%) as in table 3 & 4. (p -value = 0.03). Twelve cases have previous history of SBP, and all were culture positive. Out of which 7(58%) were MDR, 4 (33%) were XDR and 1 (8.3%) was drug sensitive as in table 5 (p-value =0.00).
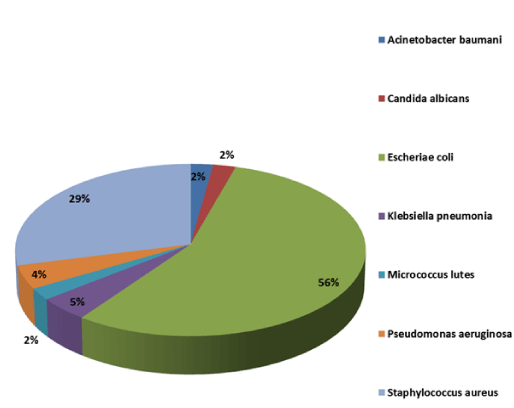
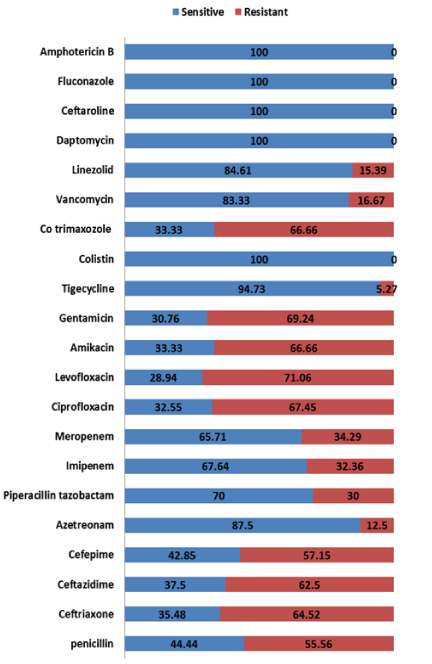
Among cases with CP-SBP; 44 (97.78%) were caused by bacteria and 1 (2.22%) was fungal. In bacterial CP-SBP, GNB were 68.19% and Gram Positive Bacteria (GPB) were 31.81%. We compared pathogen profile with resistance pattern and found significant difference among pathogens regarding resistance to antibiotics (p–value =0.00). Escheriae coli (E. coli) was cultured in 25 (55.55%) of which 12 were DS, 12 were MDR and 1 XDR; Staphylococcus aureus in 13 (28.88%) of which 10 were DS and 3 were MDR; Klebsiella pneumoniae in 2 (4.44%), both were MDR; Pseudomonas aeruginosa in 2 (4.44%) both were XDR; Acinetobacter baumanni1 (2.22%) was XDR; Micrococcus luteus in 1 (2.22%) was XDR and Candida albicans in 1 (2.22%) was DS as in table 6 and figure 1. Highest antibiotic resistance was seen with levofloxacin 71%, followed by ciprofloxacin 67.45%, cotrimoxazole 66% and ceftriaxone 64.52%. Reasonably good sensitivity in range of 70% to 90% was found in Ceftaroline, Piperacillin-tazobactam, Meropenem and Imipenem. Sensitivity was highest with Colistin, Tigecycline Daptomycin, and Ceftaroline approaching 100% as in figure 2.
Sixty six (80.49%) patient were treated successfully and discharged. Sixteen cases of SBP died during hospital stay giving mortality rate of 19.51%. Death rates as per CTP class were; 23.3% in CTP-C and 15.4% in CTP-B. There were no deaths in CTP-A.
Out of 16 deaths, 14 (71.42%) were CTP- C class. 100% deaths were recorded in patients with SBP because Acinetobacter baumani, Klebsiella pneumonia, Pseudomonas aeruginosa and Micrococcus lutes.
Discussion
SBP is one of the infections in CLD with dynamic etiologic profile demanding meticulous observation and updated management strategy in view of ever changing antibiotic sensitivity profile to improve outcome. We provide fresh insight into etiologic profile and resistance pattern in this infection.
Prevalence of SBP in CLD with ascitis presenting at our center was 38.09%. Mean age of cases was 59.09 ± 12.90 years. Most common clinical presentations were ascitis 100% and hepatic encephalopathy 89%.
Harchand P et al. [37] studied 58 cases with SBP and found prevalence of 51.3% (58/ 113). Mean age of cases was 49.72 ±10.3 years with males 86.7% and females 13.3%. Li YT et al. [27] studied 288 cases with SBP with mean age of 55 ± 12.6 years, males were 198 and females were 90. Friedrich K et al. [19] studied 311 cases of 1st time SBP with mean age of 57.8 ± 23.7 years, males were 70% males and females were 30%. Li YT et al. [27] reported 68.9%% in child C, 8.6% in child B, and 2.5% in child A class respectively in their study. Similarly studies by Kawale JB in 2017 and Harchand P in 2017 reported 56% and 87% majority of patients in CTP-C class respectively [37,38].
In our study CP-SBP was 54.87% and CN-SBP was 45.13%. Payal H Purohit et al. [39], Harchand P et al. [37] and Ivan Gunjace et al. [40] reported culture positivity of 43.6%, 38% and 15% respectively. Friedrich K. [19] reported culture positive in 138 /311 (44.37). These differences could be attributed to the evolution of pathogens, different culture techniques and variable antibiotic use. Etiological profile of SBP in CLD is changing world over. Among cases with CP-SBP; 44 (97.78%) were caused by bacteria and 1 (2.22%) was fungal (Table 6) (figure 1). Among bacterial CP-SBP, GNB were 68.19% and GPB were 31.81%. Fiore M et al. [41] reported 0%-7.2% CP for fungus and the most frequent isolate was Candida albicans. Harchand P et al. [37] reported GNB isolates in 77.3% and GPB isolates in 22.7% of cases. Ivan Gunjace et al. [40] reported GPB pathogens in 40% of patients. Payal H Purohit et al. [39] studied 31 (43.6%) cases of CP-SBP, most of them were GNB, mainly E. coli in 22 (70.9%) and GPB in 6 (19.3%) cases. Friedrich K et al. [19] reported GPB in 47.8%, GNB in 44.9% and fungal in 7.2% cultures. Li YT et al. [27] reported GPB in 27.8%, GNB in 58.2% and fungal in 2.9% cultures. Most of studies report predominance of gram negative organisms in ascitic fluid cultures, however there studies showing trend towards increasing percentage of infections caused by gram positive bacteria. Our study follows same changing trend of increasing gram positive infections world over with GPB cultures in 32% of cases.
Profile of organisms causing SBP in our study is shown in table 6 and figure 1 and their resistance profile in table 6 and figure 2. In our study, E. coli was most common organism causing SBP i.e 25/ 45 (55.55%) of which 12 were DS, 12 were MDR and 1 XDR. 52% of E. coli in our study was either MDR or XDR; Harchand P et al. [37] reported E. coli in 68.1% of cultures. Of 15 E. coli isolates, 13 (86.6%) were MDR and 3 (20%) were XDR. Friedrich K et al. [19] reported Enterobacter sp. in 41%. Li YT et al. [27] reported E. coli in 24.2% cases and drug resistance around 40%. Payal H Purohit et al. [39] in study of 31 culture-positive cases, E. coli was isolated from 17 (54.9%) cases. Ivan Gunjace et al 2010 [40] found Escherichia coli in 13 (43.6%) of cases and Li Sun et al. [42] in 53.1% (1st period part of study)/39.8% (2nd period of study) of ascitic fluid cultures respectively.
We found Staphylococcus aureus in 13 (28.88%) of which 10 were DS and 3 were MDR. Friedrich K et al. [19] reported staphylococcus sp. in 13.8%. Li YT et al. [27] reported Staphylococcus aureus in 7.5% cases. Ivan Gunjace et al. [40] cultured staphylococcus species in 4(24.9%) of cases. Li Sun et al. [42] reported Staphylococcus aureus in 4.7%/ 7.2% in 1st/ 2nd period of his study. Over all there has been increasing frequency of gram positive infection as especially staphylococcus sp. as evident above. Our study shows similar trend.
We found Klebsiella pneumoniae in 2 (4.44%) and both were MDR, Harchand P et al. [357 in 4.05%, Friedrich K et al. [19] in 7.2%, Li YT et al. [247 in 18.9%. Payal H Purohit et al. [39] isolated Klebsiella species from 5 (16.2%) cases. Li Sun et al. [42] reported Klebsiella species in 10.9%/ 10.8% in 1st / 2nd period of his study. We found Pseudomonas aeruginosa in 2 (4.44%) cases, both were XDR, Friedrich K et al. [19] in 0.7%, Li YT et al [27] in 2.9%.We found Acinetobacter baumanniin 1 (2.22%) case, it was XDR, Li YT et al. [27] in 3.3%, Ivan Gunjace et al. [40] in 2 (12.5%). our study also have more of these bacteria in HCA-SBP and HA-SBP (p value = 0.003). We found Micrococcus luteus in 1 (2.22%) was XDR and Candida albicans in 1 (2.22%) was DS. Li Sun et al. [42] found micrococcus lutes in 1.2% of case.
Results of our study are in line with current trends world over with gram negative enteric organisms most frequent isolates. Escheriae coli being most common isolate from ascitic fluid cultures in SBP patients in most of these studies. However regarding other pathogens literature is heterogeneous regarding frequency and resistance pattern.
In our study overall 71.42%, 21.42% and 7.1% isolates of gram positive bacteria were DS, MDR and XDR respectively whereas 40%, 46.6% and 13.40% isolates of gram negative bacteria were DS, MDR and XDR respectively (Table 4 & 6) (Figure2). Highest antibiotic resistance was seen with levofloxacin 71% followed by ciprofloxacin 67.45%, co-trimaxozole 66% and ceftriaxone 64.52% (Figure 2). Sensitivity was highest with Colistin, Daptomycin, and Ceftaroline approaching 100% (figure 6). Friedrich K et al. [19] found highest resistance to erythromycin 87%, ampicillin i.e 40% followed by quinolones 58%, ceftriaxone 39%, vancomycin in 49% and sensitivity was highest in Tigecycline i.e 95% followed by piperacillin/tazobactam 89.9%, gentamicin 84.3% and meropenem 73.2%. He concluded third generation cephalosporins have poor antibiotic coverage of 60% and recommends against current guidelines of third generation as first line empiric therapy. Harchand P et al. [37] found Gram-negative isolates have high susceptibility to Colistin, Tigecycline, amikacin, and Carbapenems, while low susceptibility was seen toward Cephalosporins and Ampicillin. All the Gram-positive isolates susceptible to Penicillin, Teicoplanin, Vancomycin, Linezolid whereas showed less susceptibility to Aminoglycosides (40%) and to Fluoroquinolones, Macrolides, and Tetracycline’s (20 % each). Among the culture-positive isolates, MDR and XDR were seen more in E. coli isolates. Of 15 E. coli isolates, 13 (86.6%) were MDR and 3 (20%) were XDR. Payal H Purohit et al. [39] reported Staphylococcus aureus resistance profile as; 20% in amoxicillin, 32% in erythromycin and 60% in penicillin’s: E. coli resistance profile as; 30% in ciprofloxacin, 20% in tetracycline and 10 in Ofloxacin %. Klebsiella species resistance profile reported as; 20% in ciprofloxacin, 30% in tetracycline: Pseudomonas species resistance profile as; 22% in ampicillin, 33% in ciprofloxacin, 50% in tetracycline and 20% in ofloxacin. In our study highest antibiotic resistance was seen with levofloxacin 71% followed by ciprofloxacin 67.45%, co-trimaxozole 66% and ceftriaxone 64.52% because most of these antibiotic are available in government hospital supply free of cost so are more often used. Our study shows increasing trend of GP infections and increasing MDR in gram GN infections, similarly world literature is reporting increasing incidence infections by GPB and MDR bacteria [30,34,43].
Sixty six (80.49%) patient were treated successfully and discharged. Sixteen cases of SBP died during hospital stay giving mortality rate of 19.51%. Mortality was highest in CTP-C, out of 16 cases; 14 (87.5%) belonged to CTP-C. There were no deaths in CTP-A. 100% deaths were recorded in patients with SBP because Acinetobacter baumani, Klebsiella pneumonia, Pseudomonas aeruginosa and Micrococcus lutes. Mortality in patients with SBP reported in other studies was 44% and 26.60% by Juhi B. Kawale et al. [36] and Ivan Gunjace et al. [40] respectively. Recent data reported that SBP mortality was 24.2% at 1 month and 66.5% at 3 year [16].
Conclusion
SBP in CLD with ascitis presenting at our center was 38.09%. Etiological profile of SBP in CLD is changing world over. In our study most of infections were caused by GNB (67%) and prevalence of antibiotic resistance is high approaching 60% (increasing trend). E. coli was cultured in 25 (55.55%) of which 52% were MDR’s and XDR’s; Klebsiella pneumoniae in 2 (4.44%), both were MDR; Pseudomonas aeruginosa in 2 (4.44%) both were XDR; Acinetobacter baumannii in 1 (2.22%) was XDR. High antibiotic resistance was seen to commonly used antibiotic in SBP like quinolones, aminoglycosides and third generation cephalosporins, approaching 60% to 70%. GPB were present in 31.81% of cases (increasing trend), and resistance was found in only 28.58% isolates. 50% of HCA- SBP was caused by MDR’s and 25% by XDR’s respectively i.e total of 75% (increasing trend).
We recommend all ascitic fluid culture to be screened for presence of MDR, XDR and PDR strains to improve outcome. We also suggest extended spectrum penicillin’s or Carbapenems plus vancomycin or daptomycin therapy as Ist line for suspected cases of SBP keeping in view increasing MDR pathogen infection, rising cases of community acquired MDR SBP in our community with caveat that local resistance pattern be taken into account.
- Subhas B Nadagouda, Baragundi Mahesh C, Kashinakunti SV, Birader MS. Spontaneous bacterial peritonitis of liver with ascites-across sectional study. International Journal of Biological and Medical Research. 2013; 4: 3143-3147. https://bit.ly/2pcuXHY
- Conn HO, Fessel JM. Spontaneous bacterial peritonitis in cirrhosis: variations on a theme. Medicine (Baltimore). 1971; 50: 161-197. https://bit.ly/31MesQa
- Rimola A, Soto R, Bory F, Arroyo V, Piera C, Rodes J. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology. 1984; 4: 53-58. https://bit.ly/2oicCZF
- Fernandez J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2000; 35: 140-148. https://bit.ly/362E3HP
- Agiasotelli D, Alexopoulou A, Vasilieva L, Hadziyannis E, Goukos D, Daikos GL, Dourakis SP. High serum lipopolysaccharide binding protein is associated with increased mortality in patients with decompensated cirrhosis. Liver Int.2017; 37: 576-582. https://bit.ly/2N9r4LX
- Lutz P, Goeser F, Kaczmarek DJ, Schlabe S, Nischalke HD, Nattermann J, et al. Relative Ascites Poly-morphonuclear Cell Count Indicates Bacterascites and Risk of Spontaneous Bacterial Peritonitis. Dig Dis Sci.2017; 62: 2558-2568. https://bit.ly/2PkCKOy
- Tsung PC, Ryu SH, Cha IH, Cho HW, Kim JN, Kim YS, et al. Predictive factors that influence the survival rates in liver cirrhosis patients with spontaneous bacterial peritonitis. Clin Mol Hepatol. 2013; 19: 131-139. https://bit.ly/32M0M94
- Oladimeji AA, Temi AP, Adekunle AE, Taiwo RH, Ayokunle DS. Prevalence of spontaneous bacterial peritonitis in liver cirrhosis with ascites. Pan Afr Med J. 2013; 15: 128-130. https://bit.ly/32LBaZR
- de Mattos AA, Costabeber AM, Lionço LC, Tovo CV. Multiresistant bacteria in spontaneous bacterial peritonitis: a new step in management?. World J Gastroenterol. 2014; 20: 14079-14086. https://bit.ly/2WdUKLK
- Schwabl P, Bucsics T, Soucek K, Mandorfer M, Bota S, Blacky A, et al. Risk factors for development of spontaneous bacterial peritonitis and subsequent mortality in cirrhotic patients with ascites. Liver Int. 2015; 35: 2121-2128. https://bit.ly/2BDMETk
- Kwon JH, Koh SJ, Kim W, Jung YJ, Kim JW, Kim BG, et al. Mortality associated with proton pump inhibitors in cirrhotic patients with spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2014; 29: 775-781. https://bit.ly/2WcByOu
- Nobre SR, Cabral JE, Gomes JJ, Leitão MC. In-hospital mortality in spontaneous bacterial peritonitis: a new predictive model. Eur J Gastroenterol Hepatol. 2008; 20: 1176-1181. https://bit.ly/32K9Eff
- Thuluvath PJ, Morss S, Thompson R. Spontaneous bacterial peritonitis - in-hospital mortality, predictors of survival, and health care costs from 1988 to 1998. Am J Gastroenterol. 2001; 96: 1232-1236. https://bit.ly/32OJQyA
- Runyon BA. Management of adult patients with ascites caused by cirrhosis. Hepatology. 1998; 27: 264-272. https://bit.ly/31LqpoY
- Guarner C, Soriano G. Spontaneous bacterial peritonitis. Semin Liver Dis. 1997; 17: 203-217. https://bit.ly/2MLfesw
- Alexopoulou A, Agiasotelli D, Vasilieva LE, Dourakis SP. Bacterial translocation markers in liver cirrhosis. Ann Gastroenterol 2017; 30: 486-497. https://bit.ly/31MLDmq
- Gómez-Hurtado I, Such J, Sanz Y, Francés R. Gut microbiotarelated complications in cirrhosis. World J Gastroenterol. 2014; 20: 15624-15631. https://bit.ly/2ofH9qP
- Guarner C, Soriano G. Bacterial translocation and its consequences in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2005; 17: 27-31. https://bit.ly/2PivyTd
- Friedrich K, Nüssle S, Rehlen T, Stremmel W, Mischnik A, Eisenbach C. Microbiology and resistance in first episodes of spontaneous bacterial peritonitis: implications for management and prognosis. J Gastroenterol Hepatol.2016; 31: 1191-1195. https://bit.ly/344yXsX
- Pericleous M, Sarnowski A, Moore A, Fijten R, Zaman M. The clinical management of abdominal ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: a review of current guidelines and recommendations. Eur J Gastroenterol Hepatol. 2016; 28: e10-e18. https://bit.ly/2N6Q1aM
- Strauss E. The impact of bacterial infections on survival of patients with decompensated cirrhosis. Ann Hepatol. 2013; 13: 7-19. https://bit.ly/32LCDzl
- Lutz P, Pfarr K, Nischalke HD, Krämer B, Goeser F, Glässner A, et al. The ratio of calprotectin to total protein as a diagnostic and prognostic marker for spontaneous bacterial peritonitis in patients with liver cirrhosis and ascites. Clin Chem Lab Med. 2015; 53: 2031-2039. https://bit.ly/31JxlTP
- Ison MG. Empiric treatment of nosocomial spontaneous bacterial peritonitis: One size does not fit all. Hepatology. 2016; 63: 1083-1085. https://bit.ly/2NadrMl
- Li YT, Yu CB, Huang JR, Qin ZJ, Li LJ. Pathogen profile and drug resistance analysis of spontaneous peritonitis in cirrhotic patients. World J Gastroenterol. 2015; 21: 10409-10417. https://bit.ly/34aZYLp
- Oliveira AM, Branco JC, Barosa R, Rodrigues JA, Ramos L, Martins A, et al. Clinical and microbiological characteristics associated with mortality in spontaneous bacterial peritonitis: a multicenter cohort study. Eur J Gastroenterol Hepatol. 2016; 28: 1216-1222. https://bit.ly/365XVKj
- Kamani L, Mumtaz K, Ahmed U, Ali AW, Jafri W. Outcomes in culture positive and culture negative ascitic fluid infection in patients with viral cirrhosis: cohort study. BMC Gastroenterol. 2008; 8: 59. https://bit.ly/2pQn1Mq
- Chon YE, Kim SU, Lee CK, Park JY, Kim DY, Han KH, et al . Community-acquired vs. nosocomial spontaneous bacterial peritonitis in patients with liver cirrhosis. Hepatogastroenterology. 2014; 61: 2283-2290. https://bit.ly/2p2Gdqt
- Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012; 55: 1551-1561. https://bit.ly/2WaVRMg
- Piano S, Fasolato S, Salinas F, Romano A, Tonon M, Morando F, et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: Results of a randomized, controlled clinical trial. Hepatology,2016; 63: 1299-1309. https://bit.ly/35XjrRc
- Lutz P, Nischalke HD, Krämer B, Goeser F, Kaczmarek DJ, Schlabe S, et al. Antibiotic resistance in healthcare-related and nosocomial spontaneous bacterial peritonitis. Eur J Clin Invest. 2017; 47: 44-52. https://bit.ly/2MJ9T4Q
- Ariza X, Castellote J, Lora-Tamayo J, Girbau A, Salord S, Rota R, et al. Risk factors for resistance to ceftriaxone and its impact on mortality in community, healthcare and nosocomial spontaneous bacterial peritonitis. J Hepatol. 2012; 56: 825-832. https://bit.ly/2qJatqI
- Shi L, Wu D, Wei L, Liu S, Zhao P, Tu B, et al. Corrigendum: nosocomial and community-acquired spontaneous bacterial peritonitis in patients with liver cirrhosis in china: comparative microbiology and therapeutic implications. Sci Rep. 2017; 7: 46868. https://bit.ly/2MIlnW9
- Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018; 67: 1870-1880. https://bit.ly/2NfdMO5
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pan drug resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012; 18: 268-281. https://bit.ly/31MgorP
- Harchand P, Gupta V, Ahluwalia G, Chhina RS. Clinical and bacteriological profile of spontaneous bacterial peritonitis in cirrhotic patients. J Gastrointest Infect. 2017; 7: 15-20. https://bit.ly/341TK0e
- Kawale JB, Kavita J. Rawat. Study of etiology, clinical profile and predictive factors of spontaneous bacterial peritonitis in cirrhosis of liver. Int J Res Med Sci. 2017; 5: 2326-2330. https://bit.ly/2BYFutb
- Payal H Purohit, Saklainhaider S Malek, Kairavi J Desai, Mihir Sadadia. A study of bacteriological profile of ascitic fluid in suspected clinical cases of spontaneous bacterial peritonitis at a tertiary care hospital in India. Int J Med Sci Public Health. 2015; 4: 496-501. https://bit.ly/341UAtU
- Ivan Gunjača, Ivan Francetić. Prevalence and clinical outcome of spontaneous bacterial peritonitis in hospitalized patients with liver cirrhosis: a prospective observational study in central part of Croatia. Acta Clin Croat. 2010; 49:11-18. https://bit.ly/2BIXKX8
- Fiore M, Leone S. Spontaneous fungal peritonitis: Epidemiology, current evidence and future prospective. World J Gastroenterol. 2016; 22: 7742-7747. https://bit.ly/2PiFL1H
- Li Sun, Jiu-Cong Zhang, Jun Zhao, Wen-Tao Bai, Chang-Xing Huang, Zhan-Sheng Jia, et al. Changes in the profiles of bacteria causing spontaneous bacterial peritonitis: A recent twelve-year study. African Journal of Microbiology Research. 2010; 4: 527-233. https://bit.ly/2NffHlL
- Acevedo J. Multiresistant bacterial infections in liver cirrhosis: Clinical impact and new empirical antibiotic treatment policies. World J Hepatol. 2015; 7: 916-921. https://bit.ly/2BJgGVW
- Angeloni S, Leboffe C, Parente A, Venditti M, Giordano A, Merli M, et al. Efficacy of current guidelines for the treatment of spontaneous bacterial peritonitis in the clinical practice. World J Gastroenterol. 2008; 14: 2757-2762. https://bit.ly/33VdPoK

Sign up for Article Alerts