A Study on Complications of Atrial Septal Defect (ASD) Device Closure
Goutam Datta*
Department of cardiology, Burdwan Medical College, India
*Address for Correspondence: Goutam Datta, Department of cardiology, Burdwan Medical College, India, Tel: 094-334-150-86; ORCiD: 0000-0003-3401-3101; E-mail: [email protected]
Submitted: 23 April 2020; Approved: 07 September 2020; Published: 08 September 2020
Citation this article: Datta G. A Study on Complications of Atrial Septal Defect (ASD) Device Closure. Int J Clin Cardiol Res. 2020;4(1): 031-00. https://dx.doi.org/10.37871/ijccr.id48
Copyright: © 2020 Datta G. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: ASD device closure; Complications; Mortality
Download Fulltext PDF
Introduction: Transcatheter closure of ostium secundum Atrial Septal Defect (ASD) is a safe and effective procedure. But complications like erosion, cardiac perforation, atrioventricular block, pericardial effusion, infective endocarditis, or cardiac arrhythmias may occur following ASD device closure.
Methods: One hundred patients with ostium secundum ASD were studied in a tertiary medical Centre. They were followed up for one year to assess complications of ASD device closure. Patients with significant left to right shunt (> 1.5:1), right ventricular volume overload were selected for device closure. Patients having suitable anatomy and without significant pulmonary arterial hypertension were chosen for device closure.
Results: There were sixty eight male patients and thirty two female patients in our study. Four patients had Eisenmenger syndrome. Sixteen patients went for surgical closure. Majority of our patients (twenty eight) were in thirty to forty years age group. Thirty six patients had device size of thirty to forty millimeter, thirty two patients had twenty to thirty millimeter device, twelve patients were having more than forty millimeter device. Device embolization occurred in two patients. There were four cases of pericardial effusion and pericardiocentesis was needed in two patients. Transient complete heart block was seen in two patients. Four patients had suffered from transient and self-terminating atrial arrhythmias. There was no mortality in our study.
Conclusion: Transcatheter closure of ASDs is safe and effective procedure in one year follow up study. Complications after device closure is rare. Proper selection of patients was key to the successful procedure.
Introduction
Atrial septal defects (ASD) is a common congenital heart disease and it accounts for approximately 10% of all congenital heart defects in children. The prevalence of ASD is 1 /1000 live births [1]. There are many types of ASDs-Ostium secundum, Ostium premium, Sinus venosus and Coronary sinus type of ASD. Ostium secundum type ASDs constitute for 75% of all ASDs and it is amenable to device closure. Hemodynamically significant ASD can cause right ventricular failure, atrial arrhythmia, paradoxical embolization and pulmonary arterial hypertension. The mortality rate of untreated, hemodynamically significant ASD is approximately 25% [2]. Historically, the standard treatment for patients with ASDs has always been surgical closure through a median sternotomy using cardiopulmonary bypass. The first successful surgical closure of an ASD was performed by F. John Lewis in 1952. Transcatheter closure of ostium secundum ASD is a safe and effective procedure. The first ASD device closure was performed by King and Mills in 1974. Advantages for device closure include avoidance of a sternotomy scar and cardiopulmonary bypass, less post-operative discomfort, and shorter hospital stay. The devices most commonly used are Amplatzer Septal Occluder (ASO; nickel and titanium alloy wire mesh, polyester fabric filling, US Food and Drug Administration approved in 2001) and the helex occluder (nitinol wire covered with an ultrathin polytetrafluoroethylene membrane, US Food and Drug Administration approved in 2006). But device closure is not free of complications. There may be anatomic, patient-related, and procedural variables that are associated with an increased risk of complications in patients undergoing ASD device closure. Major early adverse events occurred in 1.2% of the cases. Some of the documented risks of ASD device closure include device embolization (1%), temporary or permanent tachyarrhythmia and heart block (0.3%), erosions (0.28%), thromboembolic complications, fractures, valve injury, pericardial effusion, infections and mortality (0.05%). Device embolization is the most common adverse event (0.2%-1%) [3]. Surgical closure is preferred approach for large ASDs. There is a paucity of data on transcatheter closure of large ASDs, especially using the 40-mm devices. Our goal was to study complications of ASD device closure particularly large devices.
Methods
One hundred consecutive patients with ostium secundum ASD were studied from January, 2018 to October 2019. Study was done in a tertiary care University hospital. Study protocol was ethically approved and informed consents were taken from patients. Patients with only ostium secundum ASDs were chosen because they were amenable to device closure if indicated. Our objective was to study complications of ASD device closure. All procedures were done under conscious sedation and Transesophageal Echocardiography guidance (TEE). Balloon sizing was not done in any of the cases. All cases were selected based on both transthoracic and trans esophageal echocardiographic evaluation. Cardiac catheterization was done in twelve patients for proper assessment of pulmonary arterial pressure and pulmonary vascular resistance.
Selection criteria: Age more than eighteen years Ostium secundum ASD. Evidence of right ventricular volume overload Significant left to right shunt (> 1.5:1) No significant pulmonary arterial hypertension (pulmonary artery systolic pressure less than half of systemic arterial pressure) Anatomical suitability of device closure. Minimum follow up period was one year.
Statistical analysis: It was an prospective, observational epidemiological study. Results are expressed in absolute numbers and percentage only.
Results
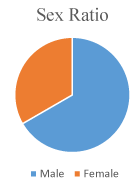
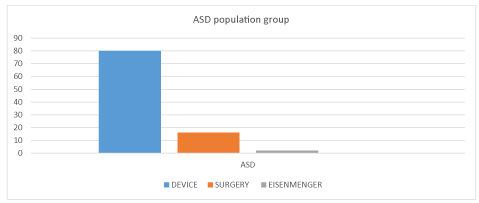
Sixty eight patients were male and thirty two patients were female. Male female ratio in our study was 2:1 (Figure 1). Four patients were suffering from Eisenmenger syndrome (4%) and they were kept on medical therapy. Sixteen patients have undergone surgical closure (16%). Twelve patients had deficient rims and they were not selected. Four patients had defect size of more than forty four mm, so they were not taken for device closure (Figure 2). Maximum device size available was 46 mm.
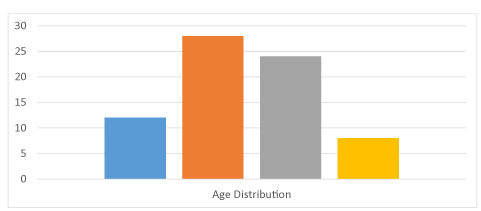
Majority of our device therapy patients (twenty eight patients) were in between thirty to forty years age group (35%). Twenty four patients were in forty to fifty six years age group (30%). There were sixteen patients in fifty to sixty years age group (20%) and twelve patients were in eighteen to thirty years age group (15%) (Figure 3).
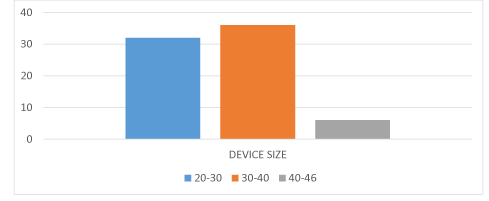
Eighty patients had undergone device closure (80%). All cases devices were deployed successfully. First patient was enrolled in Jan, 2018. Last patient was included in October, 2018. Majority of our patients had large devices. All patients were having Life Tech devices. Twelve patients had device size more than 40 mm (15%). Thirty to forty mm devices were used in thirty six patients (45%).Thirty two patients had device size less than 30 mm (40%) (Figure 4).
There was no mortality in device closure or surgically treated group. Even in our follow up of one year no patient died. We have no attrition in our follow up. But complications did happen in our procedure. There were two incidence of device embolization (2.5%). Both cases device size were 42 mm and 44 mm respectively. We have experienced two cases of transient complete heart block in peri procedure period (2.5%). Device size was 34 mm in one patient and in another case it was 38 mm. complete heart block persisted for two minutes in one patient and for eight minutes in second patient. There was spontaneous recovery in both patients but temporary pacemaker support was kept for twenty four hours. Coronary angiography was done immediately and there was no impingement on right coronary or left circumflex artery. Pericardial effusion were observed in four patients (5%) (Table 1). It was 10 -12 mm in two patents and did not require any pericardiocentesis. Effusion subsided of its own in follow up. But there were moderate to massive effusion in two patients with haemodynamic compromise. Pericardiocentesis was done immediately and both the patients recovered uneventfully. There was no recurrent pericardial collection. There were four cases of atrial arrhythmias during device closure (5%). Atrial fibrillation occurred in two patients and atrial flutter in two patients. Atrial flutter and fibrillation did not last long. All arrhythmias subsided without any pharmacological or electrical cardioversion. There was no haemodynamic instability. Only one patient had haemoglobinuria in our study (1.25%).
Discussion
Atrial septal defect is a common congenital heart disease with incidence of 1.0/1,000 live births. 4Atrial septal defects are classified into ostium primum, ostium secundum, sinus venosus and coronary sinus types. ASD constitutes about 10% of congenital heart diseases. Among the various types of ASDs only the ostium secundum defect is amenable to device closure. All four types can be closed surgically. King and Mills, in 1974, originally described feasibility of ASD device closure [5].
Untreated ASD can cause right ventricular overload with right heart failure, atrial arrhythmias, pulmonary arterial hypertension, systemic embolism and death. Transcatheter closure has now become the standard treatment strategy for ostium secundum ASD if there is suitable anatomy. A recent study comparing 4,606 percutaneous procedures and 3,159 surgical ASD closures at 35 children’s hospitals in USA showed that transcatheter closure was as safe as surgery and provided better short-term value when compared with surgical closure [6].
Device closure is indicated for ostium secundum ASD patients if rims are suitable and there is significant left to right shunt (> 1.5:1) without any evidence of significant pulmonary arterial hypertension (pulmonary artery systolic pressure less than half of systemic arterial pressure). Right ventricular volume overload indicates significant left to right shunt. There are six rims which are evaluated by echocardiography. These are superior, inferior, aortic, posterior, superior vena cava and inferior vena caval rims. Ideally all rims should be present and at least five millimeter in length. But in cases of large ostium secundum ASD retro aortic rim is not adequate in many times [7].
Large ASD is usually defined as when defect size is more than ten millimeter. But for device closure, any device whose size is more than thirty millimeter is taken as large device [8]. In our study large devices were deployed in forty eight patients which was sixty percent of our patients. Twenty four patients had deficient retro aortic rims which is thirty percent of our ASD device group. The prevalence of deficient aortic rim varies between 11.4 to 60% [9]. Mc Elhinney et al reported a 24% prevalence of deficient aortic rim in their control patients [10].
Incidence of erosion is around .043-.3% [11]. Deficient retro aortic rim and oversized devices are two important predisposing factors for erosion. Erosion usually occurs within one to three months of device deployment. Erosion will lead to fistula formation and it may communicate with different cardiac chambers. It is a lethal complication of device closure and necessitates surgical intervention. Rarely late erosion may occur. In our study we did not experience any case of erosion in one year follow up though many of our patients had large devices with deficient retro aortic rims. Twenty eight cases of erosion with hemodynamic compromise were reported between 1998 and March 2004 in USA. Erosion rate in USA was 0.1% (9 of 9,000 known U.S. implants) [12]. Aortic or superior rim was deficient in twenty five patients. Amongst twenty eight patients: Five involved perforation at the roof of the left atrium and the aorta; six had perforation at the roof of the right atrium and the aorta; in one case, both atria were involved; in three cases, there was aortic perforations. Nineteen patients had symptoms within 72 hrs of device deployment. In eight patients, diagnosis was made between five days and eight months of procedure. Pericardial effusion due to perforation developed after three years in one patient. Sixteen had device removal in addition to perforation or fistula repair during surgery. The device was kept in five patients because these were in optimal position but the perforation was repaired by surgery [13]. Seven patients had responded to conservative treatment. The signs and symptoms of erosion include cardiac tamponade, pericardial effusion, hemodynamic compromise, chest pain, shortness of breath, syncope, and sudden cardiac death.
Complete heart block was seen in two patients in our study. Both the cases complete heart block was transient. Mechanism of complete heart block in ASD device closure is unknown. It could be due to stretching of interatrial septum which may interfere with Atrio Ventricular (AV) nodal conduction time. Large device may impinge on right coronary artery or rarely left circumflex artery which are supplying AV nodal artery. The risk of bundle branch block in patients with large ASD, particularly patients with deficient rims, may be increased, A retrospective study of six hundred and ten device closure patients showed clinically significant heart block occurring in 0.3% of patients [14].
ASD devices consist of two discs made of nitinol (nickel and titanium alloy) mesh. Patients may experience nitinol allergy. None of our patients experienced that. Reaction may occur from 2 days up to 1 month after implantation. It may manifest as headaches, rash, urticaria, difficulty in breathing, fever, or pericardial effusion. It usually responds to medical management. In rare instances, if medical management fails, the devices may need to be explanted [15].
There were no incidence of air embolism, thrombus formation or infection in our study. The FDA analyses of the MAUDE Medical-Device Reports (MDR) showed 0.8% incidence of infection or endocarditis [16].
Haemoglobinuria may occur in device closure patients. This is due to turbulence at the device site. Haemoglobinuria occurred in one of our patient and device size was thirty six millimeter [17].
Device embolization is a known phenomenon. Incidence varies between 1 to 3% [18]. Proper evaluation of rims by transesophageal echocardiography is extremely important. Failure of rim assessment and large device size are two important factors for device embolization. Percutaneous retrieval is possible in many cases but surgical help should be sought in failed cases. In our study it happened in two patients. Both the cases we failed to retrieve them percutaneously. Devices size were 42 mm and 44 mm respectively. They went for surgical retrieval and closure successfully. In one case inferior vena caval rim was not adequate and other case Eustachian valve was mistaken as inferior vena caval rim. These were surgical finding in two patients. There were twenty one device embolizations out of 3,824 implants (0.55%) in USA in 2003. Fifteen were retrieved using a transcatheter approach (71.4%) and six were retrieved surgically (28.5%) [19]. In another study device was retrieved surgically in 77.2% of cases and by transcatheter approach in 16.7% of cases. There were 2 deaths related to embolization [20].
There were four cases of pericardial effusion in our study. Pericardiocentesis was required in two patients. Mechanisms of pericardial effusion is unclear. It could be due to terumo wire induced perforation of pulmonary vein or left atrial appendage. Terumo wire was used to cross the defect and to guide delivery sheath to pulmonary veins. Device erosion was another possibility. Cardiac CT was done in all four cases to find out the site of perforation but nothing could be found in post-operative period.
Atrial arrhythmia was observed in perioperative period in four patients. Two cases of atrial flutter and two cases of atrial fibrillation were seen in our study. All of them were self-terminating and did not produce any haemodynamic instability. These are may be due to guidewire, sheath or device induced irritation of atrial wall. In the MAUDE analysis, arrhythmias were seen in five percent of patients. . There is a concern that device closure of ASD may preclude future electrophysiology procedures that require transseptal access [21].
Conclusion
Transcatheter closure of ASDs is safe and effective procedure in one year follow up study. Complications after device closure is rare. There was no mortality in our study. Proper assessment of rims and defect size are key to success.
Limitations
It is an observation study. Study population size is small. Both type 1 and type 2 error may occur in interpretation as it is a small single center study.
- Gary Webb, Michael A Gatzoulis. Atrial septal defects in the adult: Recent progress and overview. Circulation. 2006; 114: 1645-1653. DOI: 10.1161/CIRCULATIONAHA.105.592055
- Ooi YK, Kelleman M, Ehrlich A, Glanville M, Porter A, Kim D, et al. Transcatheter versus surgical closure of atrial septal defects in children: A value comparison. JACC Cardiovascular Intervention. 2016; 9: 79-86. DOI: 10.1016/j.jcin.2015.09.028
- Alban Elouen Baruteau, Jerome Petit, Virginie Lambert, Marielle Gouton, Dominique Piot, Philippe Brenot, et al. Transcatheter closure of large atrial septal defects: Feasibility and safety in a large adult and pediatric population. Circ Cardiovasc Interv. 2014; 7: 837-843. DOI: 10.1161/CIRCINTERVENTIONS.113.001254
- Julien I E Hoffman, Samuel Kaplan. The incidence of congenital heart disease. J Am Coll Cardiol. 2002; 39: 1890-900. DOI: 10.1016/s0735-1097(02)01886-7
- T Podnar, P Martanovic, P Gavora, J Masura. Morphological variations of secundum-type atrial septal defects: Feasibility for percutaneous closure using Amplatzer septal occluders. Catheter Cardiovasc Interv. 2001; 53: 386-391. DOI: 10.1002/ccd.1187
- Kutty S, Hazeem AA, Brown K, Danford CJ, Worley SE, Delaney JW, et al. Long-term (5- to 20-year) outcomes after transcatheter or surgical treatment of hemodynamically significant isolated secundum atrial septal defect. Am J Cardiol. 2012; 109: 1348-1352. DOI: 10.1016/j.amjcard.2011.12.031
- Gerard R Martin, Robert H Beekman, Frank F Ing, Kathy J Jenkins, Chuck R McKay, John W Moore, et al. The IMPACT registry: IMproving Pediatric and Adult Congenital Treatments. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010; 13: 20-25. DOI: 10.1053/j.pcsu.2010.02.004
- Jones TK, Latson LA, Zahn E, Fleishman CE, Jacobson J, Vincent R, et al. Results of the US. multicenter pivotal study of the helex septal occluder for percutaneous closure of secundum atrial septal defects. J Am Coll Cardiol. 2007; 49: 2215-2221. DOI: 10.1016/j.jacc.2006.11.053
- Byrne ML, Glatz AC, Sunderji S, Mathew A, Goldberg D, Dori Y, et al. Prevalence of deficient retro-aortic rim and its effects on outcomes in device closure of atrial septal defects. Pediatr Cardiol. 2014; 35: 1181-1190. DOI: 10.1007/s00246-014-0914-6
- Mc Elhinney DB , Quartermain MD , Kenny D, Alboliras E, Amin J . Relative risk factors for cardiac erosion following transcatheter closure of atrial septal defects: A case-control study. Circulation. 2016; 133: 1738-1746. DOI: 10.1161/CIRCULATIONAHA.115.019987
- Johanne Auriau, Helene Bouvaist, Lars Aaberge, Tadaaki Abe, Ingo Dahnert, Joseph Panzer, et al. Cardiac erosions after transcatheter atrial septal defect closure with the occlutechfigulla flex device. JACC Cardiovasc Interv.2019; 12:1397-1399. DOI: 10.1016/j.jcin.2019.03.005
- Daniel J DiBardino, Doff B McElhinney, Aditya K Kaza, John E Mayer Jr. Analysis of the US food and drug administration manufacturer and user facility device experience database for adverse events involving Amplatzer septal occluder devices and comparison with the society of thoracic surgery congenital cardiac surgery database. J Thorac Cardiovasc Surg. 2009; 137: 1334-1341. DOI: 10.1016/j.jtcvs.2009.02.032
- Everett AD, Jennings J, Sibinga E ,Owada C, Lim S ,Chetham J et al. Community use of the Amplatzer atrial septal defect occluder: Results of the multicenter MAGIC atrial septal defect study. Pediatr Cardiol. 2009; 30: 240-247. DOI: 10.1007/s00246-008-9325-x
- Shada J Al Anani, Howard Weber, Ziyad M Hijazi. Atrioventricular block after transcatheter ASD closure using the Amplatzer septal occluder: Risk factors and recommendations. Catheter Cardiovasc Interv. 2010; 75: 767-772. DOI: 10.1002/ccd.22359
- Brent M Gordon, John W Moore. Nickel for your thoughts: Survey of the Congenital Cardiovascular Interventional Study Consortium (CCISC) for nickel allergy. J Invasive Cardiol. 2009; 21: 326-329. PubMed: https://pubmed.ncbi.nlm.nih.gov/19571342/
- Dong Jun Kim, Chi Young Shim, Seng Chan You, Seung Hyun Lee, Geu Ru Hong. Late bacterial endocarditis and abscess formation after implantation of an amplatzer septal occluder device. Circulation. 2015; 131: 536-538. DOI: 10.1161/CIRCULATIONAHA.115.016339
- Zakaria Jalal, Sebastien Hascoet, Alban Elouen Baruteau, Xavier Iriart, Bernard Kreitmann, Younes Boudjemline, et al. Long-term complications after transcatheter atrial septal defect closure: A review of the medical literature. Can J Cardiol. 2016; 32: 1315-1318. DOI: 10.1016/j.cjca.2016.02.068
- Daniel S Levi, John W Moore. Embolization and retrieval of the Amplatzer septal occlude. Catheter Cardiovasc Interv. 2004; 61: 543-547. DOI: 10.1002/ccd.20011
- Massimo Chessa, Mario Carminati, Gianfranco Butera, Roberta Margherita Bini, Manuela Drago, Luca Rosti, et al. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol. 2002; 39: 1061-1065. DOI: 10.1016/s0735-1097(02)01711-4
- Alessia Faccini, Gianfranco Butera. Atrial septal defect (ASD) device trans-catheter closure: Limitations. J Thorac Dis. 2018; 10: 2923-2930. DOI: 10.21037/jtd.2018.07.128
- Jonathan N Johnson 1, Michelle L Marquardt, Michael J Ackerman, Samuel J Asirvatham, Guy S Reeder, Allison K Cabalka, et al. Electrocardiographic changes and arrhythmias following percutaneous atrial septal defect and patent foramen ovale device closure. Catheter Cardiovasc Interv. 2011; 78: 254-261. DOI: 10.1002/ccd.23028

Sign up for Article Alerts