Migration of ApoA1+ Macrophage Phenotypes from Pericoronary Adipose Tissue to Coronary Plaque: A Possible Mechanism for Suppression of Human Coronary Atherosclerosis
Nobuyuki Hiruta1, Yasumi Uchida2,3*, Ei Shimoyama4 and Tsuyoshi Tabata5
1Department of Pathology, Toho University Sakura Medical Center, Sakura, Japan 2Japanese Foundation for Cardiovascular Research, Funabashi, Japan 3Department of Cardiology, Tokyo Jikei University School of Medicine, Tokyo, Japan 4Department of Pathology, Funabashi-Futawa Hospital, Funabashi, Japan 5Department of Clinical Physiology, Toho University Sakura Medical Center, Sakura, Japan
*Address for Correspondence: Yasumi Uchida, Japanese Foundation for Cardiovascular Research, Funabashi, Japan; Department of Cardiology, Tokyo Jikei University School of Medicine, 2-30-17, Narashinodai, Funabashi, Japan, 274-0063, Tel: +814-746-221-59; E-mail: uchida73@ta2.so-net.ne.jp
Submitted: 24 June 2020; Approved: 07 July 2020; Published: 08 July 2020
Citation this article: Hiruta N, Uchida Y, Shimoyama E, Tabata T. Migration of ApoA1+ Macrophage Phenotypes from Pericoronary Adipose Tissue to Coronary Plaque: A Possible Mechanism for Suppression of Human Coronary Atherosclerosis. Int J Clin Cardiol Res. 2020;4(1): 012-020.https://dx.doi.org/10.37871/ijccr.id46
Copyright: © 2020 Hiruta N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Apolipoprotein A1; Human coronary plaques; Immunohistochemical staining; Macrophage phenotypes; Pericoronary adipose tissue
Download Fulltext PDF
Background: Apolipoprotein A1 (ApoA1) is an important anti-atherogenic protein. We previously found that ApoA1 is stored in human Pericoronary Adipose Tissue (PCAT) and macrophages residing in PCAT accumulate ApoA1. Here, we aimed to identify whether and how the macrophage phenotypes transport ApoA1 from PCAT to the atherosclerotic coronary plaques.
Methods: Coronary arteries and their surrounding PCAT were excised from human autopsy subjects and examined using immunohistochemical techniques to investigate macrophage-mediated ApoA1 transport from PCAT to the coronary intima.
Results: CD68+ and CD206+ macrophages containing ApoA1 were observed in PCAT of both normal coronary segments (normal group) and coronary segments with plaques (plaque group).
External Elastic Lamina (EEL) was loosened or fragmented, and Internal Elastic Lamina (IEL) was disrupted in the plaque group. ApoA1-containing CD68+ and CD206+ macrophages (M2 macrophages), extending pseudopod forward, were frequently observed to pass through these portions into the intima. ApoA1-containing CD11c+ macrophages (M1 macrophages) were not found in PCAT, EEL, or media, but were found in disrupted IEL and plaques.
Conclusions: The results suggest that CD206+ or CD68+ macrophages transport ApoA1 from PCAT into the media and they and CD11c+macrophages transformed at the site of IEE into plaque via loosened or fragmented EEL and disrupted IEL and could participate in the suppression of coronary atherosclerosis.
Abbreviations
ApoA1: Apolipoprotein A1; EEL: External Elastic Lamina; IEL: Internal Elastic Lamina; oxLDL: oxidized Low-Density Lipoprotein; PCAT: Pericoronary Adipose Tissue
Introduction
It is a general belief that an important anti-atherogenic substance, Apolipoprotein A1 (ApoA1), is considered as in case of High-Density Lipoprotein (HDL), enters into the vascular wall from the vascular lumen to act against atherosclerosis by mediating reverse cholesterol transport [1-3].
Previously, we found that ApoA1 is stored in human Pericoronary Adipose Tissue (PCAT) and accumulated in macrophages residing in the PCAT [4].
Using immunohistochemical techniques, normal coronary segments (normal group) or segments containing plaques (plaque group) together with their surrounding PCAT, from human autopsy subjects who had been diagnosed with ischemic heart disease, we investigated which of atherosclerosis-related macrophage phenotypes, i.e., CD68+ (a nonspecific marker of macrophages), CD11c+ (a marker of atherogenic M1-macrophages) [5,6] and CD206+ (a marker of anti-inflammatory and antiatherogenic M2-macrophage), [5] obtain ApoA1 from PCAT and through what route(s) they transport ApoA1 to the intima (plaque) of the adjacent coronary artery.
Methods
Angioscopic and immunohistochemical studies of excised human Pericoronary Adipose Tissue (PCAT) and the adjacent coronary artery
Ethics: The procedures followed were in accordance with the “Declaration of Helsinki” and the ethical standards of the Toho University Ethical Committee and Ethical Committee of Funabashi-Futawa Hospital on human experimentation, and after obtaining written informed consent from the families involved regarding the use of excised coronary artery and its surrounding adipose tissue for angioscopic and histological studies to clarify the mechanisms underlying atherosclerosis.
Subjects: The proximal to middle segments of coronary arteries (7 left anterior descending arteries and 7 right coronary arteries) and the surrounding adipose tissue were carefully excised from 7 autopsy cases of patients with coronary artery disease who had died at Funabashi-Futawa Hospital or Toho University Medical Center Sakura Hospital [61.0 ± 8.3 years (mean ± SD); 2 females and 5 males]. Three had an acute myocardial infarction, two had an old myocardial infarction, and the remaining two patients had angina pectoris. Causes of death were: pneumonia (2), congestive heart failure (1), diabetic nephropathy (1), cerebral infarction (1), visceral carcinoma (1), and sudden death (1) (Table 1).
Classification of coronary plaques and normal segments by conventional angioscopy and histology
Here, a conventional coronary angioscopy system (details described elsewhere [7,8]) was used to classify coronary plaques and normal segments.
By conventional coronary angioscopy, a plaque is defined as a nonmobile, protruding or lining mass that is clearly demarcated from the adjacent normal wall and had a shape, location and color that does not change after saline flushing. A normal segment is defined as a milky-white and smooth-surfaced portion without any protrusions [9]. Classification of plaques and normal segments by angioscopy was performed independently using images recorded on DVD disks by two observers who did not participate in the conventional angioscopy procedure. An angioscopic normal segment was considered to be included within a plaque when the intimal thickness was found to exceed 300µm in histological sections prepared for single immunohistochemical staining.
Observation of excised coronary arteries by conventional angioscopy
A Y-connector was introduced into the proximal portion of the respective coronary artery for perfusion with saline solution at a rate of 20 mL/min, subsequently, an angioscope was introduced through the connector into the artery to evaluate it for plaques and normal segments. Disrupted plaques were excluded. Plaques or normal segments could be identified because the light irradiated from the tip of the angioscope was visible through the arterial wall [10].
Selection of plaques and normal segments: Sections (4-5 -mm in length) of proximal or middle segments of the anterior descending coronary artery or right coronary artery and the surrounding PCAT were isolated by transecting the proximal and distal ends at the shorter axes.
Normal segments and those with plaques were excised (one each from each subject); thus, 7 normal segments (normal group) and 7 segments with plaques (plaque group) were obtained and used for further examination (Table 1).
Excised segments were embedded in O.C.T. Compound (Sakura Finetek USA Inc., Torrance, CA) before being stored at -20°C. Before segments were embeded, a 0.5 mm section was obtained from each segment and fixed with 5% glutaraldehyde for electron microscopy.
Definition of PCAT: The epicardial adipose tissue located within 3 mm (nearly the same as the diameter of the proximal to middle segments of the coronary artery) of the EEL of an epicardial coronary artery was arbitrarily defined as PCAT because such tissue may more likely to directly influence the coronary artery than more remotely located epicardial adipose tissue.
Double immunohistochemical staining: All plaques and normal segments with their surrounding PCAT, which had been stored at -20°C, were cut into successive 20 µm sections on a cryostat (Tissue Tec 3D, SakuraFinetec Japan, Tokyo). Such relatively thick and frozen sections were used to prevent leaking from the PCAT. Sections were fixed with 4 % paraformaldehyde for 7 min at 4°C, and incubated with a mixture of 1% hydrogen peroxide in methanol for 30min. Successive sections were processed by double immunohistochemical staining as follows: ApoA1 with CD68 for nonspecific macrophages [11], ApoA1 with CD11c for M1-inflammatory and atherogenic macrophages [12] and ApoA1 with CD206 for M2- anti-inflammatory and anti-atherogenic macrophages [13].
The antibodies used for immunohistochemical staining are as follows:
For ApoA1, Anti-apoA1-antibody orb 10973; rabbit polyclonal, which reacts with human apoA1; Biorbyt Ltd, Cambridge, UK.
For CD68, anti-CD68 antibody mouse monoclonal NCL-CD 68-KP1, Leica Biosystems Newcastle Ltd, Newcastle, UK [11].
For CD11c, an anti-CD11c antibody (ab52632, rabbit monoclonal (EP1347Y), reacts with human CD11c, Abcam Ltd).
For CD206, a human MMR/CD206 antibody, source polyclonal goat IgG, immunogen mouse myeloma cell line NSO-derived recombinant human MMR/CD206, R&D Systems, Minneapolis, MN
Sections were incubated with anti-CD68, anti-CD206 or anti-CD11c antibodies for 60 min Subsequently, the sections were incubated with anti-mouse Alexa555 (Alexa Fluoro555 goat anti-mouse IgG, Code A21422, Molecular Probe Ltd, CA, USA) for 30 min to emit a red fluorescence for CD68, CD206 or CD11c. The same sections were incubated with the anti-ApoA1 antibody for 60 min, and then with anti-rabbit FITC (FITC conjugated Affeini Pure goat anti-rabbit IgG, Code 111-095-003, Vector Laboratories Inc, Burlingame, CA, USA) for 30 min to emit a green fluorescence for ApoA1. Finally, the section was reacted with DAPI (4’, 6-diamidino-2-phenylindole; Life Technologies Carlsbad, Carsbad, CA, USA) to emit a blue fluorescence for cell nuclei [14].
Stained sections were photographed separately or merged with one another using a microscope (IX70, Olympus Co, Tokyo, Japan) connected through an ICCD camera (DP 73, Olympus Co) to CellSens Standard (Olympus Co). For fluorescence imaging, a 460 nm band-pass filter (BPF) and a 510 nm Band Absorption Filter (BAF) for the green fluorescence of ApoA1, a 555 nm BPF and a 575 nm BAF for the red fluorescence of macrophages, and a 345 nm BPF and 420 nm BAF for the blue fluorescence of cell nuclei as previously reported [15].
Density of ApoA1-containing (ApoA1+) macrophage phenotypes in PCAT and intima
Using double-immunohistochemically stained samples, the density of ApoA1+ macrophage phenotypes (per 200 µm x 200µm area2) was counted in PCAT, media and intima and compared between normal and plaque groups. Areas where cell nuclei, which exhibit blue spots, were distributed most densely by densitometry were selected for counting.
Microscopic observation of EEL and IEL
EEL and IEL exhibit a strong fluorescence when stained with fluorescein. One section from each sample was stained with fluorescein, after which EEL and IEL were examined using fluorescent microscopy. Normal EEL is composed of tight continuous elastin fibers that form a band. When any loosening or fragmentation was noted, EEL was defined as loosened or fragmented EEL, and the number of loosened or fragmented portions in the circumference of EEL was compared between the normal and plaque groups.
IEL is composed of elastin plates arranged in series connected side- by-side with thin filaments. In a normal coronary artery, the distance between the plates is ≦ 5 µm [16]. Therefore, widening of the distance by 10 µm or fractured or decaying portions of IEL were defined as disruption. The number of disrupted portions across the circumference of the IEL was compared between the normal and plaque groups.
The number of ApoA1-containing (ApoA1+) macrophage phenotypes passing through the EEL or IEL
The number of ApoA1-containing macrophage phenotypes passing through the circumference of the EEL or IEL was counted and compared between the normal and plaque groups. Macrophages are amoeboid cells and extend pseudopod toward the direction of crawling. Therefore, pseudopod side was considered as the direction of crawling.
Electron microscopic study
Because macrophages are amoeboid in shape, cells with an amoebic configuration and migrating through the IEL were examined using electron microscopy (InTouchScopeTM, JSM-IT200, Nihondenshi Co., Tokyo, Japan).
Statistical analysis
Fisher’s exact test was used for statistical analysis of data. The data obtained were expressed as mean± standard deviation (SD), a p-value of < 0.05 was considered to be statistically significant. Because of a large number of comparisons, the Bonferroni correction was used [17].
Results
ApoA1 in PCAT
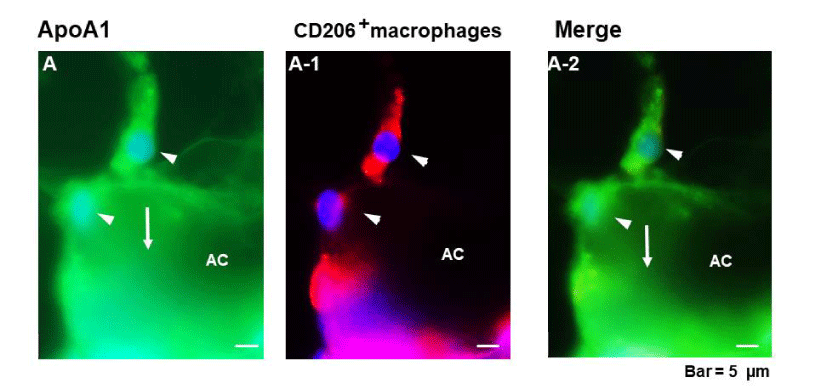
ApoA1 was present in Adipocyte (AC) cytoplasm (arrow in figure 1; A) and in CD206+macrophages (arrowheads in figure 1; A A-2). These patterns were observed in the majority of samples of the normal and plaque groups.
ApoA1+ macrophages
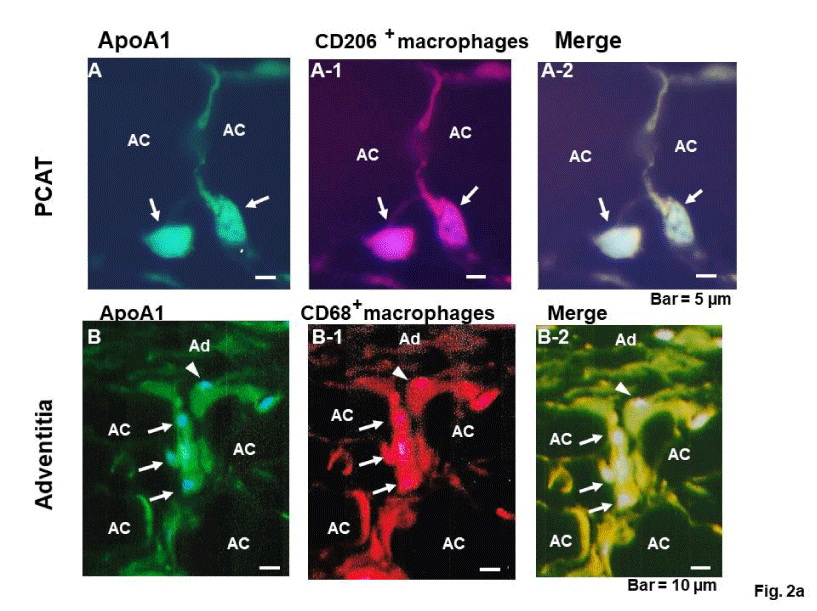
Figure 2a and 2b show double immunohistochemical staining of ApoA1 which is contained in macrophage phenotypes in PCAT, adventitia, media and intima. ApoA1+macrorophage phenotypes were CD68+ or CD206+ in PCAT. They resided in the interstitial space between adipocytes (arrows in figure 2a; A to A-2).
The adventitia and PCAT are not clearly margined, forming a border zone, and these two macrophage phenotypes appear to migrate through the interstitial space of PCAT to the adventitia (Figure 2a; B to B-2).
In the media, these two phenotypes are eel-like in configuration and resided along with circular smooth muscle cells (Figure 2b; A to A-2).
In the plaque, CD68+, CD206+ but also CD11c+ macrophages that contain ApoA1 were observed. They were either spindle-like or round in configuration (Figure 2b; B to B-2). About a half to one-thirds of macrophages did not contain ApoA1. Fragmented particles of ApoA1-containing foam cells were also observed.
The density of ApoA1+CD68+ and ApoA1+CD206+ macrophages in the PCAT of normal and plaque groups exhibited no notable difference (Figure 2c; A). ApoA1+ CD11c+macrophages were not found in PCAT (Figure 2c; A).
The density of ApoA1+ CD68+-and ApoA1+CD206+ macrophages in the media was higher in the plaque group than the normal group. ApoA1+ CD11c+ macrophages were not found in the media (Figure 2d; B). The density of ApoA1+CD68 (+)-, and ApoA1+CD206 (+) macrophages in the intima (plaque) was higher in the plaque group (Figure 2c; C). ApoA1+CD11c+ macrophages, which were not observed in PCAT and media, were now observed in plaques (Figure 2c; C).
Differences in the External Elastic Lamina (EEL) and Internal Elastic Lamina (IEL) morphology between normal and plaque groups
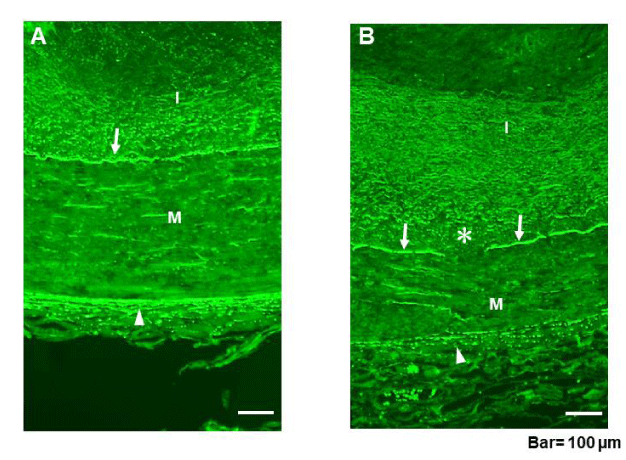
In the normal group, the EEL was tight and continuously arranged (arrowheads in figure 3a; A). The IEL, which is composed of elastin plates in series, was wavy and almost continuously arranged (arrow in figure 3a; A).
However, in the plaque group, the EEL was fragmented (arrowhead in figure 3a; B) and the IEL was frequently disrupted (asterisk in figure 3a; B).
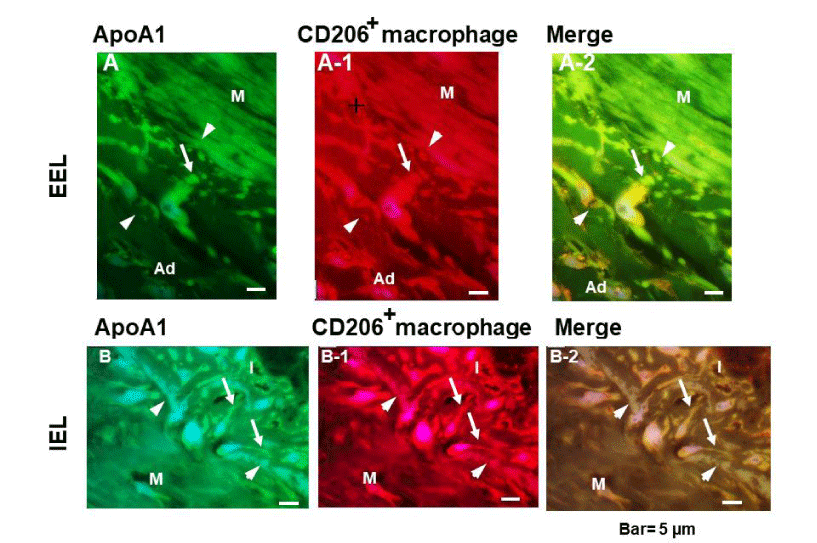
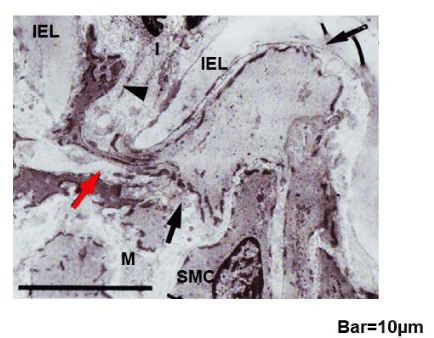
Crawling of ApoA1+ macrophages through the EEL and IEL
Figure 3b shows ApoA1+ macrophages migrating through fragmented EEL (A to A-2), and through the disrupted portions of IEL (B to B-2). Pseudopod protruding toward the media (arrow in A) and toward the intima (arrows in B), indicating direction of their crawling.
Electron microscopy of macrophage-like cells crawling through the IEL
Cells that were amoebic in configuration, which is characteristic of macrophages, protruding pseudopod through the disrupted IEL into the intima were frequently observed in plaque group (arrowhead in figure 3c), indicating that they had only begun to enter into the intima.
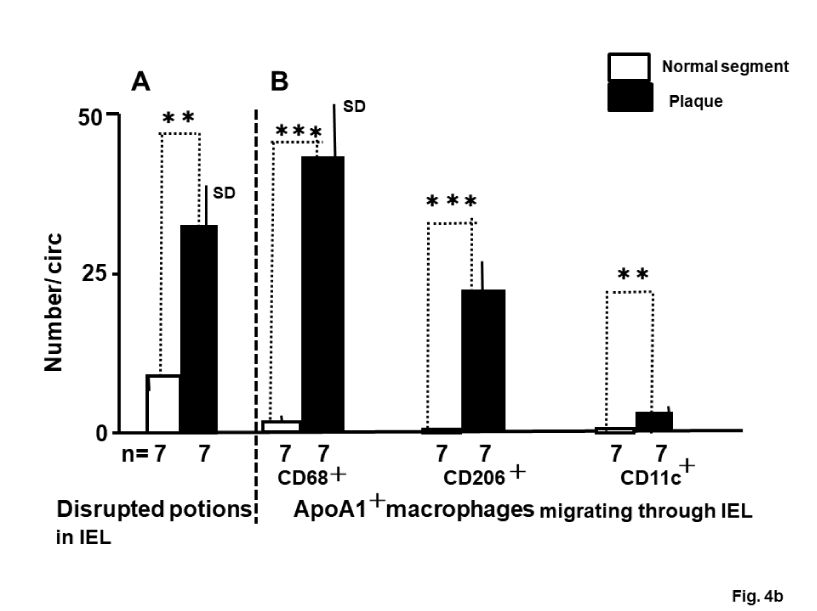
Incidence of loosened or fragmented EEL or disrupted IEL and migration of ApoA1+ macrophages through EEL and IEL
The incidence of loosened or fragmented portions in the EEL and disrupted portions in the IEL was significantly higher in the plaque group than in the normal group (Figure 4a; A, Figure 4b; A).
The incidence of ApoA1+ CD68+ and ApoA1+CD206+macrophages migrating through the EEL was more frequent in the plaque group than in the normal group (Figure 4a; B). The incidence of these macrophages migrating through disrupted portions of the IEL was also higher in the plaque group than in the normal group (Figure 4b; B). Notably, ApoA1+CD11c (+) macrophages were observed in the disrupted portion of the IEL in plaque group but were not observed in the IEL of the normal group (Figure 4b; B).
Discussion
ApoA1+macrophage phenotypes in PCAT
Storage of ApoA1 was observed in PCAT not only in the plaque group but also in the normal group, indicating that ApoA1 storage begins before atherosclerosis development.
The density of ApoA1+CD68+ and ApoA1+CD206+ macrophages in PCAT between the normal and plaque groups was not different, indicating that the ApoA1-containing capacity of these macrophages was not influenced by the presence or absence of plaques in the adjacent coronary intima.
Mechanism(s) for damages in the EEL and IEL
Loosened or fragmented EEL and disrupted IEL were frequently observed in coronary segments with plaques (plaque group). There are at least three possible mechanisms for such changes: (1) Plaques induce distension of the EEL or IEL, which subsequently damages them. In a normal coronary artery, the IEL is composed of elastin Plates [18] in series, which are connected side by side with thin filaments, and the space between the plates is less than 5 µm [16]. The filaments connecting the IEL plates were likely disrupted, and the plates became separated as a result of plaque distension. (2) Because the EEL and IEL are composed primarily of elastin, elastases that diffused from the lumen or adventitia damaged the EEL and IEL [18]. (3) Macrophages-excreted cytokines during macrophage migration caused the damage to the EEL and IEL [19-21].
Migration of ApoA1+ macrophage phenotypes through the EEL and IEL
The incidence of ApoA1+ macrophages migrating through the EEL and IEL was higher in the plaque group. The EEL and IEL were unable to function as a mechanical or humoral barrier and may have played a role in enhancing macrophage migration.
Direction of macrophage migration
Ameba and amoeboid cells such as white blood cell and macrophages protrude a pseudopod forward, retracts the pseudopod, changes body shape and crawls, indicating that the pseudopod shows the direction of crawling. In this study, ApoA1+ macrophage phenotypes in the loosened ELL or disrupted IEL protruded their pseudopod toward the media and toward the intima, respectively, strongly suggesting that they crawled toward the media and then to the intima [22-24].
Macrophage transformation
There are many macrophage phenotypes. Among them, M1-macrophages (CD11c+ macrophages) are considered as atherogenic and M2-macrophages (CD206+ macrophages) are considered antiatherogenic, primarily based on findings in animals or cultured cells [25]. In this study, CD11c+macrophages were not found in PCAT, adventitia or media, but were found at the sites of disrupted IEL, suggesting that CD68+- or CD206+ macrophages switched phenotype to CD11c+ macrophages during the migration through the IEL.
In the present study, ApoA1 was found in both CD206+ and CD11c+ macrophages. This finding suggests that CD11c+ and CD206+ macrophages (so-called M1 - and M2 -macrophages, respectively) are not well differentiated concerning ApoA1 carriage in man. Switching of macrophages from M2 - to M1 - is seen in animals, [26] and a similar switch might have occurred in the human macrophages during migration or because the macrophages may have possessed both M1 and M2 characteristics, and the potency of these characteristics changed while traversing the IEL. Further studies are necessary to clarify the roles of individual macrophage phenotypes, including the subtypes of M1-macorphages [27], and M2-macropahges [25], concerning ApoA1 carriage.
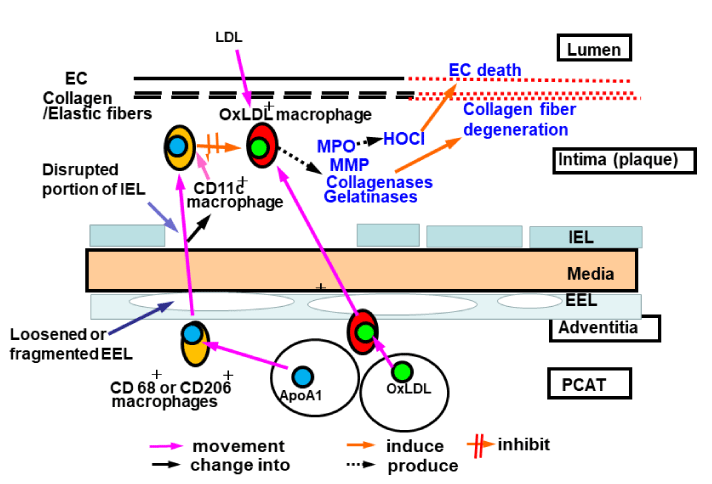
Possible mechanisms for the migration of ApoA1+ macrophages from PCAT to the intima
Based on the findings of the present study, we propose a possible mechanism for the prevention of human coronary atherosclerosis. The ApoA1 stored in PCAT is transferred to CD68+ and CD206+ macrophages that reside in the interstitial space between adipocytes; ApoA1+ CD68+ and/or ApoA1+ CD206+macrophages migrate through the adventitia and loosened or fragmented EEL into the media. Subsequently, these macrophages migrate through the disrupted portions of the IEL, and a percentage of the migrating macrophages transform into CD11c+ macrophages, and enter into the intima (plaque). The ApoA1+ macrophage phenotypes entered into the intima (plaque), interact with atherogenic substances or cells including oxidized Low-Density Lipoprotein (oxLDL) or oxLDL-containing macrophages [28,29] and suppress atherosclerosis (Figure 5).
Study Limitations
(1) Because a majority of the autopsy cases were admitted for a serious condition at the terminal stage, lipid plasma levels and other substances examined before death were not considered to be reflective of the patient’s the levels during stable conditions. Therefore, it was difficult to examine the association of plasma lipid levels and other substances with macrophage phenotypes and behaviors.
(2) Although ApoA1 storage in human PCAT was confirmed, the following mechanisms remain to be elucidated;
(a) The factor(s) that regulate ApoA1 storage in PCAT as well as macrophage migration into the intima (plaque) remain to be elucidated.
(b) It remains unknown whether ApoA1 is synthetized in PCAT, or if it is transported to PCAT from an unknown origin.
Clinical perspectives
Enhancing ApoA1 storage in PCAT and its macrophage-induced transport to the plaque may contribute to the prevention or regression of coronary atherosclerosis. Therapies enhancing storage of ApoA1 in PCAT or migration of ApoA1+macrophages could contribute in preventing human coronary atherosclerosis.
Conclusion
ApoA1 is stored in human PCAT. CD68+ and CD206+macrophages acquire ApoA1 from PCAT and transport it through the adventitia, loosened and fragmented EEL, media, and disrupted IEL into the intima (plaque). However, a percentage of these macrophages are converted to CD11c+macrophages during migration through the IEL before entering into the intima. Our findings suggest that such an “outside-in” process could contribute to the suppression of human coronary atherosclerosis.
Author contributions
Yasumi Uchida and Nobuyuki Hiruta conceived and designed the study, performed the in vitro study and wrote the manuscript; Ei Shimoyama and Nobuyuki Hiruta performed the autopsies and immunohistochemical staining and conducted microscopic studies; and Tsuyoshi Tabata performed the statistical analysis. All authors participated in editing the manuscript.
- Boeckold SM, Arsenault BJ, Hovingh GK, Mora S, Pedersen TR, Larosa JC, et al. Levels and changes of HDL cholesterol and apolipoprotein A-1 in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation. 2013; 128: 1504-1512. DOI: 10.1161/CIRCULATIONAHA.113.002670
- Emoto T, Sawada T, Morimoto N, Tenjin T, Wakimoto T, Ikeda F, et al. The apolipoprotein B/A1 ratio is associated with reactive oxygen metabolites and endothelial dysfunction in statin-treated patients with coronary artery disease. J Atheroscler Thromb. 2013; 20: 623-629. DOI: 10.5551/jat.16824
- Pan L, Lu G, Chen Z. Combined use of apolipoprotein B/apolipoprotein A1 ratio and non-high-density lipoprotein cholesterol before routine clinical lipid measurement in predicting coronary heart disease. Coron Artery Dis. 2014; 25: 433-438. DOI: 10.1097/MCA.0000000000000100
- Uchida Y, Uchida Y, Shimoyama E, Hiruta K, Tabata T, Kobayashi T. Deposition patterns and localization of apolipoprotein A1 and their relation to plaque morphology in human coronary artery. JSM Atherosclerosis. 2017; 2: 1025-1034.
- Lee CW, Hwang I, Park CS, Lee H, Park DW, Kang SJ, et al. Macrophage heterogeneity of culprit coronary plaques in patients with acute myocardial infarction or stable angina. Am J Clin Pathol 2013; 139: 317-322. DOI: 10.1309/AJCP7KEYGN3OBGQX
- Cho KY, Miyoshi H, Kuroda S, Yasuda H, Kamiyama K, Nakagawara J, et al. The phenotype of infiltrating macrophages influences arteriosclerotic plaque vulnerability in carotid artery. J Stroke Cerebrovasc Dis 2013; 22: 910-918. DOI: 10.1016/j.jstrokecerebrovasdis.2012.11.020
- Uchida, Y. Angioscopy system. In: Atlas for Cardioangioscopy. Uchida Y, editor. Tokyo: Medical View Ltd; 1995. p. 23-27.
- Uchida Y. Angioscopes and its manipulation. In: Uchida Y, editor. Coronary angioscopy. Armonk, NY: Futura Publishing LTD; 2001. p. 11-24. https://bit.ly/3e49LaA
- Uchida Y. Clinical classification of atherosclerotic coronary plaques. In: Coronary angioscopy. Uchida Y (editor). Armonk, NY: Futura Publishing Ltd; 2001. p. 71-81. https://bit.ly/3e49LaA
- Uchida Y, Uchida H, Kawai S, Shirai S, Tomaru T, Maezawa Y. Detection of vulnerable coronary plaques by color fluorescent angioscopy. JACC Cardiovasc Imaging. 2010; 3: 398-408. DOI: 10.1016/j.jcmg.2009.09.030
- Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993; 18: 1607-1613. PubMed: https://pubmed.ncbi.nlm.nih.gov/7680921/
- McAlpine CS, Huang A, Emdin A, Banko NS, Beriault DR, Shi Y, et al. Deletion of myeloid GSK3 alpha Attenuates atherosclerosis and promotes an M2 macrophage Phenotype. Arterioscler Thromb Vasc Biol. 2015; 35: 1113-1122. DOI: 10.1161/ATVBAHA.115.305438
- Park SJ, Lee KP, Kang S, Lee J, Sato K, Chung HY, et al. Shingosine 1- phosphate induced anti-atherogenic and atheroprotective M2 macrophage polarization through IL-4. Cell Signal. 2014; 26: 2249-2258. DOI: 10.1016/j.cellsig.2014.07.009
- Bonne, D., Heusele, C., Simon, C., & Pantaloni, D. 4’,6-Diamidino-2-phenylindole, a fluorescent probe for tubulin and microtubules. J Biol Chem. 1985; 260: 2819-2825. PubMed: https://pubmed.ncbi.nlm.nih.gov/3972806/
- Uchida, Y. Uchida Y, Shimoyama E, Hiruta N, Kishimoto T, Watanabe S. Human pericoronary adipose tissue as storage and possible supply site for oxidized low-density lipoprotein and high-density lipoprotein in coronary artery. J Cardiol. 2017; 69: 236-244. DOI: 10.1016/j.jjcc.2016.03.015
- Uchida Y, Uchida Y, Matsuyama A, Koga A, Maezawa Y, Maezawa Y. Functional medial thickening and folding of the internal elastic lamina in coronary spasm. Am J Physiol Circ Physiol. 2011; 300: H423-430. DOI: 10.1152/ajpheart.00959.2010
- Armstrong RA. When to use the Bonferroni correction. Ophthalmoc Physiol Opt. 2014; 34: 502-508. DOI: 10.1111/opo.12131
- Osbome-Pellegrin MJ, Farijanel J, Homebeck W. Role of elastase and lysyl oxidase in spontaneous rupture of internal elastic lamina in rats. Atherosclerosis. 1990; 10: 1136-1146. DOI: 10.1161/01.atv.10.6.1136
- Denswil NP, van der Wal AC, Ritz K, de Boar OJ, Aronica E, Troost D, et al. Atherosclerosis in the circle of Willis: Spacial differences in composition and in distribution of plaques. Atherosclerosis. 2016; 251: 78-84. DOI: 10.1016/j.atherosclerosis.2016.05.047
- Sugiyama S, Kuriyama K, Aikawa M, Nakamura S, Ogawa H, Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque and thrombogenesis. Arterioscler Thromb Vasc Biol. 2004; 24: 139-145. DOI: 10.1161/01.ATV.0000131784.50633.4f
- Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018; 233: 6425-6440. DOI: 10.1002/jcp.26429
- Wessels DJ, Kuhl S, Soll DR. Light microscopy to image and quantify cell movement. Methods Mol Biol. 2009; 571: 455-471. DOI: 10.1007/978-1-60761-198-1_30
- Friedl P, Borgmann S, Broecker EB. Amoeboid leukocyte crawling through extracellular matrix: lessons from the Dictyostelium paradigm of cell movement. J Leuko Biol. 2001; 70: 491-509. PubMed: https://pubmed.ncbi.nlm.nih.gov/11590185/
- Antrovic V, Marinovic M, Filic V, Weber I. A simple optical configuration for cell tracking by dark-field microscopy. J Microbiol Methods. 2014; 104: 9-11. DOI: 10.1016/j.mimet.2014.06.006
- DePaori F, Staeles B, Chinetti-Ghaguidi G. Macrophage phenotypes and their modulation in atherosclerosis. Circ J 2014; 78: 1775-1787. DOI: 10.1253/circj.cj-14-0621
- Kallou-Lachest J, Varthuaman A, Fornasa G, Compain C, Gaston AT, Clement M, et al. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010; 5: e8852. DOI: 10.1371/journal.pone.0008852
- Gambaro SE, Zubiria MG, Portales AE, Rey MA, Rumbo M, Giovambattista A. M1 macrophages subtypes activation and adipocyte dysfunction worsen during prolonged consumption of a fructose-rich diet. J Nutr Biochem. 2018; 61: 173-182. DOI: 10.1016/j.jnutbio.2018.08.004
- Libby P. The vascular biology of atherosclerosis. In: Mann DL, Zipes DP, Libby P, Bonow RO, Braunwald E, editors. Heart disease 10th ed. Philadelphia: Elsevier Saunders Ltd; 2015; 873-890.
- Yoshida H, Kisugi R. Mechanisms of LDL oxidation. Chimica Acta. 2011; 411: 1875-1882. DOI: 10.1016/j.cca.2010.08.038




Sign up for Article Alerts