Spinal Cord Stimulation Costs and Complications can be reduced by Wireless Nanotechnology. A Review of Traditional Equipment Expenses Compared to Wireless Stimulation?
Laura Tyler Perryman*
Stimwave Technologies, Inc. 901 Las Olas Blvd., Suite 201, Fort Lauderdale, FL 33301, USA
*Address for Correspondence: Laura Tyler Perryman, Stimwave Technologies, Inc. 901 Las Olas Blvd., Suite 201, Fort Lauderdale, FL 33301, USA, Tel: +800-965-5134; E-mail: [email protected]
Submitted: 19 October 2018; Approved: 22 November 2018; Published: 28 November 2018
Citation this article: Laura Tyler Perryman. Spinal Cord Stimulation Costs and Complications can be reduced by Wireless Nanotechnology. A Review of Traditional Equipment Expenses Compared to Wireless Stimulation. Am J Anesth Clin Res. 2018;4(1): 019-024.
Copyright: © 2018 Laura Tyler Perryman. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Keywords: Spinal cord stimulation; Chronic pain; Implantable power generator; Costs; wireless
Download Fulltext PDF
Background: Spinal Cord Stimulation (SCS) has been a cost-effective therapeutic approach for controlling chronic pain, following spinal surgery, peripheral neuropathy, complex regional pain syndromes and others. However, the surgically implantable nature of Traditional SCS (TSCS) components not only increased the surgical complications but also the costs associated with the device. Wireless SCS (WSCS) access to the implanted nanoelectrode can reduce the bulk of the equipment thus improving patient acceptance with fewer surgical complications as well as low costs.
Objective: Review of the literature on costs of TSCS compared to the costs of novel SCS with wireless technology.
Material and Results: A review of the available limited literature on TSCS costs show that implantation incurred USD 32,882 (CAD 21,595 and UK £ 15,081) while expenditure for WSCS was 18,000 Euro. Further analysis revealed that costs for a nonrechargeable battery was USD 13,150 (CSD 10,591; UK £ 7,243) in 2006 while a rechargeable battery had cost USD 20,858. Maintenance costs for the SCS equipment included a battery change every 4 years, on an average costing USD 3,539. IPG replacement involved expenses of CAD 5.071. A wireless device (Stimwave) is devoid of IPG costs and required a 3-year maintenance costs of 1500 Euros only.
Additionally, the Wireless SCS (WSCS) was equally effective and without the added complications of IPG that included pocket area pain, hematoma (10%) and infection (50% of infections following TSCS implantation). Management of IPG complications costs additional health care budget, while with WSCS, this could be an avoidable burden. WSCS has been reported to be as effective as TSCS in management of chronic pain following back surgery, herpes infection and complex regional pain syndrome in case illustrations.
Conclusions: SCS has been an established tool in effective management of chronic pain. TSCS equipment costs more and includes IPG costs between 13,000 and 20,000 USD with a maintenance expense of 3,539 USD over 4 years (for battery change) while WSCS had been reported to have nearly half of this maintenance cost for SCS therapy and without IPG costs and complications.
Introduction
Chronic pain remains to be a major factor driving people to seek relief and is a major consumer of opioid medication as well as research resources. The gate theory for pain mechanisms proposed by Melzak and Wall was quickly followed by introduction of Spinal Cord Stimulation (SCS) therapy by Shealy and his team from Cleveland breaking ground for the field of neuromodulation in the management of chronic intractable pain [1,2].
Literature has time and again established the cost effectiveness of SCS in the management of chronic pain due to failed back surgery syndrome, neuropathic pain disorders, complex regional pain syndromes as well as vascular ischemia [3-8].
With proven efficacy SCS is being increasingly utilized and the number of indications as well as surgeries performed annually have been on the rise [9]. Rapid advancements have refined the equipment to improve the longevity of the treatment as well as the implants. However, there are very few reports on the burden the present day SCS technology carries in terms of costs, comfort and complications (Table 1).
The costs of traditional SCS implant
Traditionally, SCS equipment utilizes implantable electrode enclosed inside a catheter, long extension wires connecting these electrodes to an IPG and all these components are placed surgically inside the patient body. Thus, complications following the surgical implantations as well as the failures of any of these components are by default considered as the consequences of Neuromodulation therapy. There are instances where the trial SCS fails and in some instances SCS fails to achieve the therapeutic goal. Complications following SCS implantation have been reported in up to 35 to 40% patients [5,10-12].
The costs of SCS therapy include, implantation, maintenance and complications. However, most places take only implantation costs in to consideration and a lot of times the costs of complications, which can be very fluctuating, are not included. Incidentally, the incidence of complications on an average could be seen in one third of cases and some of them turn out to be expensive to manage [5,13,14] and the SCS therapy, in general, faces accountability from third party payers.
For calculating budgets in cases where implants are placed, costs must include initial implantation charges, annual maintenance costs and the expenses incurred to treat complications. The maintenance costs might seem to be stable in uncomplicated cases, but once a complication sets in, the annual maintenance burden increases and thus each case might be allowed an 18% increase in the budget [3,4].
Cost of SCS implantation
Kumar et al. [15] reported the North American experience from Canada and the US (Medicare), covering the costs of consultation, diagnostic procedures, trial stimulation and implantation. The Canadian health care system charges CAD 21,595 for implantation of the SCS equipment while the US had a mean cost of USD 32,882 (Medicare). The cost of trial SCS was CAD 7671 and USD 10900 (Medicare). The Canadian expenses included a longer hospital stay (of 3 days) since most patients travel nearly 1000 miles to reach the specialized care centers and stay hospitalized unlike the US pain management centers.
Maintenance costs
The annual maintenance costs of SCS were CAD 3539 and USD 5071 for uncomplicated cases. Since the nonrechargeable IPG needed replacement after 4 years, the costs for IPG and maintenance were amortized for 4 years [15].
Three papers described the life expectancy of an IPG [7,16,17] and average life of an IPG was 49 months considering the best figures. Kumar et al reported a mean life expectancy of IPG as 48 months in a review of 104 patients [17], while Van Buyten had just 27.9 months (32 of 61 patients required IPG replacement during a follow up of 5 years [7]. Considering the other 29 patients, the mean life time of IPG was 50 months. Budd reported on fewer patients only with only 18 months follow up [16]. The life span of an IPG is an important issue in the calculation of SCS expenditure, since many complications of SCS have been attributed to this implantable design of the battery and the IPG needs replacement once it reaches end-of-life.
Electrode migrations and IPG sites
Displacement of electrodes is related to the stabilization capacity of the anchor and the tensile load carried by the electrode which varies by the mobility of the spine, the tethering effect of IPG and the elastic nature of the tissues around the electrode [15]. Much of the excursions of the cables along with the attached lead change by the spine movements and the site of IPG implantation. There is experimental data demonstrating that about 9cm of displacement happens between thoracic spine and buttock IPG location with flexion-extension movements of the thoracolumbar spine. It was much less when the IPG is placed in the anterior abdominal wall. The electrode displacement was 0.2 cm with walking and 1.7 cm with twisting of the trunk while these valued doubled when IPG was in the gluteal area compared to abdominal wall locations [18]. A strain loop recommended to reduce these excursions might be helpful until scar tissue encases the loops and tethers the wiring during normal movements. In cases of scoliosis there might be 2-fold increased lateral displacement in the thoracolumbar region and in the cervical region due to the natural neck movements. Paddle electrodes in such circumstances would reduce the displacement. Quadripolar and octapolar electrodes have also reduced incidence of lead migrations. North et al reported a reduction in revision rates for lead migration to 16% with multichannel devices compared to 23% with simple bipolar leads [19]. Alo et al had only 3.8% cases going for surgical revision when octapolar leads were implanted [20].
Costs of rechargeable or nonrechargeable IPG
The initial implantation costs of a rechargeable IPG are high, while there has been an increasing trend to utilize this type of battery even though the difference between a rechargeable and a nonrechargeable IPG is very significant. A rechargeable IPG is costlier by CAD 10,591 (USD 10,988) just for implantation [15] because of the cost of equipment, which again differs among the manufacturing companies and by state in the US. The maintenance costs also might be different since the life span of the rechargeable system varies by the company: a Medtronic product lasts for 9 years, Advances Neuromodulation Systems (St. Jude or Abbott) for 7 years and Boston Scientific battery for 5 years.
The engineering team from Boston Scientific, however reported the rechargeable battery life could vary between 10 and 25 years [21]. Accordingly, the maintenance costs also differ by the length of battery life as a rechargeable IPG requires 2-3 replacements while a nonrechargeable IPG might need 5-6 reimplantation procedures. Additionally, there are other factors like age of the patient at the time of SCS, tolerance of the therapy and complications influencing the expenses and the cost benefit [16,21,22].
Costs of complications during SCS therapy
Surgical complications are expensive, and the costs depend upon the hospitalization, implants and the resources utilized to treat the patients [21,22]. A surgical infections costs about CAD 18,837 (includes antibiotic treatment, explantation and reimplantation of a new system) compared to aspiration of subcutaneous collection/hematoma (CAD 136) and the mean cost of a complications following SCS in Canada was CAD 5191, while in the US it measures up to USD 9649 (ranging between 381 and 28,495).
Individualized complication costs for patient
Kumar et al encountered 63 adverse events in their experience with 51 patients (1.23 per patient) with a mean cost of a complication per patient as CAD 556 (USD 1034 for the Medicare patient in the US). The total cost of complications over 3.65 years was CAD 327,057 for all the 161 patients, escalating the annual maintenance costs 1.4 times in Canada and twice in the US [23].
Cost of complications
Mean expenses for a patient: Costs of SCS complication management vary depending upon the severity: a minor aspiration of subcutaneous pocket collection costs minimal compared to an infected device which requires thorough investigations and explantation followed by reimplantation of the system [15]. Kumar et al reported a mean cost of a complication to be CAD 7092 (ranging between 130 and 22,406) with a maintenance cost of CAD 3609 (includes IPG replacement once in 4 years) for an uncomplicated case while the system implantation incurs CAD 23,205. A failed trial SCS cost CAD 7859 per patient, with explantation charges of an additional CAD1739 per case. If these cases and the failed SCS therapy instances were put together the mean costs of SCS increased to CAD 24,809.
Repositioning of the leads had cost 360 Euro, while replacing a lead and infection indicating reimplantation had cost 1530 and 6192 Euro respectively [23]. Similar complications in the US required USD 2700, USD 5450 and 19,600 (lead repositioning, lead replacement and infection) while a IPG failure and replacement cost 13,150 USD [24]. There is a learning curve reported by many and in the experience of Kumar et al there was a 5% decrease in surgical revisions for SCS complications after the first 10 years [25].
A complicated vs an uncomplicated case: An uncomplicated case of SCS is much less expensive to manage. Kumar et al. [15,22] reported costs amounting to CAD 3609 to cover the physician consult, medications, nursing management and adjusted expenses for IPG replacement calculated for every 4 years. However, once complications set in (reportedly in as many as 40% of the cases) the annual maintenance dollars increase almost by three times compared to the uncomplicated cases. Kumar et al calculated an average expenditure of CAD 7092 on complicated SCS management. Initial implant cost was CAD 23,205 and a case without any complications required only one tenth of this cost for maintenance over 4 years while it was CAD 10,701 on an average with complications [15].
Percutaneous and paddle electrode systems and the costs: A percutaneous procedure has the advantage of being minimally invasive, outpatient procedure offering lower costs compared to surgical placement of a paddle electrodes although the latter system offers lower rates of lead migrations but higher complications [26]. Reoperation rates among paddle electrode are always significantly fewer indicating that the newer SCS devices might be successful in reducing lead fracture and migrations. On the other hand, both types of electrode systems utilized similar amount of health care resources over 2 year follow up period in terms of in-patient and outpatient emergency care as well as medication, However, the percutaneous systems had significantly higher out-patient costs (USD 100,486) compared to paddle electrode systems (USD 87,961) at 2 years and also at 5 years (USD 186,139 and 169,768). High Frequency SCS costs and maintenance: In a model created by Annemans et al. [27], High Frequency SCS (HF SCS) was more cost effective and provided better quality therapy compared to TSCS and SCS with nonrechargeable battery. These authors reported a complication rate of 14.4% with all these three modalities of SCS and replacement rate varied between 3.94% and 7.25%. The costs for complication management was £ 622.
Neuromodulation with wireless nanotechnology
Conventional SCS technology utilizes implantable electrodes enclosed inside a catheter, lengthy extension cables connecting to an IPG; all of them surgically implanted inside the patient body and complications related to these procedures as well as the failures of the equipment components are translated as failure of the Neuromodulation therapy. Research efforts to improve the efficacy yielded IPG with longer life expectancy and reduced size, but not with less expenditure or surgical trauma. Advancement in this field is the new external Wireless Power Generator (WPG) that applies a dipole antenna for electric field coupling. This is accomplished via the very short-length pulsed Electromagnetic (EM) waves known as ‘microwaves’ at Giga Hertz frequencies (GHz). This wireless device (Stimwave technologies), instead of lower inductive frequencies (ranging between 100-500 kHz) for most of the implanted medical devices, is powered by Radiative electric field coupling through tissues at microwave frequencies [28].
These microwaves enable miniature sized implants to be placed at a significantly deeper tissues through a needle or by minimally invasive procedure and yet accessible wireless. The higher frequencies applied for stimulation, afford only minimal power loss and on the other hand offer superior energy transfer to even smaller sized implants This energy transfer phenomenon was mentioned earlier by Feynman, Father of Nanotechnology, as the principle behind the frequency vs wavelength changes in his introductory talk on nanotechnology (there is plenty of room at the bottom) and accordingly skin depth only decreases with square root of the scale ratio (scale on which frequency goes up and wavelength comes down). As he mentioned in this presentation, superconductors today have reduced the resistance in modern physics [29].
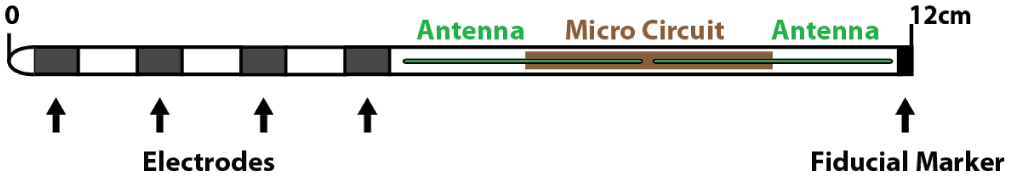
The nano-material implant in the WPG, capable of delivering the clinically appropriate range of stimulation at 800-1350 um diameter, is a very small sized implant compared to the conventional SCS-IPG. Additionally, the amperage requirements for Dorsal Root Ganglion (DRG) stimulation compared to SCS are much lower thus increasing the longevity of the WPG. The implant size is equivalent to the standard lead body of the SCS and incorporates the Nano electronics within itself. It can be included in to different contact types of leads (4 or 8 contacts) suitable for both percutaneous cylinder type or paddle type electrodes. The receiver wire is mated to the implant to communicate with the external/wireless power generator (Figure 1).
An oscillating electric field is created as the dipole antenna receiver intercepts the microwave EM frequencies emanated from the EPG. The antenna within the device can vary between 2cm and 8cm in length (with modifications possible depending upon the depth of implantation). The EM energy can be dissipated at variable depths starting from skin to bone across the intervening fat, muscle, blood vessels. Previous experimental models demonstrated that frequency at GHz range were more energy efficient [30]. The animal models showed that deeper placements require longer antenna to receive the require power. As part of an application specific integrated circuit, each contact on the stimulating lead is provided with exclusive power capabilities, since the circuits inside the contacts produce very specific charge balanced waveforms (Figure 2).
The WPG in place of implantable power generators
Just like the present-day cellular phones, WPG employs a similar transfer technology. The average pulse output of power is up to 1 Watt depending upon the required stimulation and the depth at which the stimulator is placed. The WPG has a Radiofrequency (RF) transmitter that transforms the stimulation waveforms in to a signal as per the program setting given by the clinician or the patient; while a microprocessor within the transmitter regulates the settings and data transfers (Figure 3). A controller utilizing Bluetooth technology makes it convenient for both the patient and the clinician to access the WPG for suitable modifications; which can also be performed via an app on a cellular phone [28]. Additionally, neuronal damage is very less likely with EM wave forms at micro wavelengths; since high frequency does not activate the cell membranes. Thus, the wireless nanotechnology device not only affords minimally invasive surgical implant, but the microwave energy is more compatible with biological safety also.
To reduce the complications associated with the bulk of the conventional SCS equipment, many modifications have been proposed. The most important upcoming innovation is wireless access to the implant to provide Neuromodulation. In several clinical conditions, wireless nanotechnology stimulation system has been utilized for SCS, DRGS and PNS throughout Europe and in the USA over the past couple of years with encouraging responses, albeit in case studies.
The operating capabilities of wireless power transmission in biological media have been demonstrated at GHz range (as against the MHz of the conventional stimulation methods), by Poon et al offering potential advantages [30,31]. There was a remarkable reduction in the size of the receiver at this frequency. Subsequent studies by Tyler Perryman et al demonstrated the relationships between tissue depth and energy transmission in animal models [32]. The author conducted experimental studies in pig models to verify the tissue depth and the accessibility of the wireless transmission of signals to achieve effective current density [32]. In this study, at 915 MHz, the dipole antenna of the WPG could energize the nanostimulators placed at the depth of 12 cm in porcine models; an antenna of 4.3 cm was more efficient.
In clinical scenario, successful wireless stimulation and significant pain relief was observed in patients with back pain, leg pain, neuralgia following herpes zoster, craniofacial pain, occipital neuralgia, and complex regional pain syndrome [32-36]. Adverse events or complications in these short case series and reports were minimal. The wireless access by the WPG, to the nanoelectode required implantation of the stimulating electrode (with embedded sensors) only; excluding additional surgical trauma from implantation of IPG and its accessories. Thus, complications related to these components were avoided. There was reduced surgical trauma, operating time, usage of consumables with increased comfort and cosmetic result to the patient.
Costs involved with nanotechnology wireless SCS are much less comparatively: The initial implantation of the wireless electrode costs 18,000 Euro and does not have any costs related to IPG or its accessories. The annual maintenance expenses for the wireless system are approximately 1500 Euro/3 years. This procedure involves no additional surgery or hospitalization for battery failures or explantation or re-implantation (Table 2).
For obvious reasons, since WSCS is devoid of IPG, there was no battery/extension cable related complications and costs. Additionally, in the long run with increased patient numbers, the wireless neuromodulation technology can be expected to yield far better outcomes, fewer complications, improved cosmetic results and very much reduced costs.
Limitations
As a novel technology wireless SCS needs more case material and further experience to provide detailed information on costs of complications and device related adverse events. The cost might come down with widespread usage and expanded indications. Unfortunately, there are only few reports on the costs of traditional SCS implantation, its complications and long-term maintenance. Additional publications on audits might provide better insights in to the cost effectiveness of SCS since so far it has been compared to conservative medical management only.
Summary
SCS has been a time tested, cost-effective neuromodulation modality in the management of chronic intractable pain with rapid technological advancements improving the trial success rate as well as long term outcomes. Only few reports, however came up with the comforts, costs and challenging complications of SCS so as to improve the future applications. With the advent of wireless nanotechnologies minimizing the device dimensions along with surgical trauma, it is time to reevaluate the expenditure a TSCS carries and its burden on the health care budget. Certainly, the recent advanced implants models appear to mitigate or reduce the complications as well as the costs associated with the traditional devices, especially by avoiding the IPG costs and complications. Implantation of a single miniature device has the promising advantages of less tissue trauma, fewer surgical procedures, minimal implant, reduced hospital stay, lower complication rate and fewer follow up visits translating in to a significantly low cost with equally efficient outcome.
- Melzack R, Wall PD. Pain mechanisms. A new theory. Science. 1965; 150: 971-979. https://goo.gl/HSfTRQ
- Shealy S, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967; 46: 489-491. https://goo.gl/bxmmK1
- Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in the treatment planning and present status, a 22 year experience. Neurosurgery. 2006; 58: 481-496, https://goo.gl/KGPCcN
- Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008; 63: 762-770. https://goo.gl/Gt9GWx
- North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005; 56: 98-106. https://goo.gl/yU29ag
- Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome: results of a systematic review and meta-analysis. J Pain Symptom Manage. 2006; 31: S13–S19. https://goo.gl/1nLJ4k
- Van Buyten JP. The performance and safety of an implantable spinal cord stimulation system in patients with chronic pain: a 5-year study. Neuromodulation. 2008; 6: 79-87. https://goo.gl/LifrdR
- Manca A, Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain. 2008; 12: 1047-1058. https://goo.gl/VaUvaF
- Praeger J. Estimates of annual spinal cord stimulator implant rises in the United States. Neuromodulation. 2010; 13: 68-69. https://goo.gl/VKeVZA
- Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004; 100(3 Suppl Spine): 254-267. https://goo.gl/xdRTJU
- Kumar K, Buchser E, Linderoth B,Meglio M, Van Buyten JP. Avoiding complications from spinal cord stimulation: practical recommendations from an international panel of experts. Neuromodulation. 2007; 10: 24-33. https://goo.gl/fAiQKH
- Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation. 2015; 18: 603-609. https://goo.gl/zTqSvQ
- Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomized controlled trial in patients with failed back surgery syndrome. Pain. 2007; 132: 179-188. https://goo.gl/N3MvmE
- Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004; 108: 137-147. https://goo.gl/69e6XS
- Kumar K, Wilson JR, Taylor RS, Gupta S. Complications of spinal cord stimulation, suggestions to improve outcome and financial impact. J Neurosurg Spine. 2006; 5: 191-203. https://goo.gl/m2TSUz
- Budd K. Spinal cord stimulation: cost-benefit study. Neuromodulation. 2002; 5: 75-78. https://goo.gl/pGhsDC
- Kumar K, Malik S, Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: cost-effectiveness analysis. Neurosurgery. 2002; 51: 106-116. https://goo.gl/5LqvZi
- Sakas DE, Simpson BA. Operative Neuromodulation. Acta Neurochirurgica. Supplement 97, 2006. https://goo.gl/gURdg1
- North RB, Ewend MG, Lawton MT, Piantadosi S. Spinal cord stimulation for chronic, intractable pain: superiority of “multichannel” devices. Pain. 1991; 44: 119-130. https://goo.gl/jkPWZQ
- Alo KM, Yland MJ, Kramer DL, Charnov JH, Redko V. Computer assisted and patient interactive programming of dual octrode spinal cord stimulation in the treatment of chronic pain. Neuromodulation. 1998; 1: 30-45. https://goo.gl/B4RBYa
- Hornberger J, Kumar K, Verhulst E, Clark MA, Hernandez J. Rechargeable spinal cord stimulation versus non-rechargeable system for patient with failed back surgery syndrome: a cost consequences analysis. Clin J Pain. 2008; 24: 244-252. https://goo.gl/YZy8HW
- Kumar K, Bishop S. Financial impact of spinal cord stimulation on the health care budget: a comparative analysis of costs in Canada and the United States. J Neurosurg Spine. 2009; 10: 564-573. https://goo.gl/WhAXth
- Kemler MA, Furnee CA. Economic evaluation of spinal cord stimulation for chronic reflex sympathetic dystrophy. Neurology. 2002; 59: 1203-1209. https://goo.gl/caa5zR
- Bell GK, Kidd D, North RB. Cost-effectiveness analysis of spinal cord stimulation in treatment of failed back surgery syndrome. J Pain Symptom Manage. 1997; 13: 286-295. https://goo.gl/X1kpD5
- Kumar K, Nath R, Wyant GM. Treatment of chronic pain by epidural spinal cord stimulation: a 10-year experience. J Neurosurg. 1991; 75: 402-407. https://goo.gl/QKrWRd
- Babu R, Hazzard MA, Huang KT, Ugiliweneza B, Patil CG, Boakye M, et al. Outcomes of percutaneous and paddle lead implantation for spinal cord stimulation: a comparative analysis of complications, reoperation rates, and health-care costs. Neuromodulation. 2013; 16: 418–427. https://goo.gl/hoYXpP
- Annemans L, Van Buyten JP, Smith T, Al-Kaisy A. Cost effectiveness of a novel 10 kHz High-Frequency spinal cord stimulation system in patients with Failed Back Surgery Syndrome (FBSS). J Long Term Eff Med Implants. 2014; 24: 173-183. https://goo.gl/QSw2RK
- Yearwood TL, Perryman LT. Peripheral neurostimulation with a microsize wireless stimulator. In. Slavin (Ed) Stimulation of the peripheral nervous system. The Neuromodulation Frontier. Prog Neurol Surg Basel Karger. 2016; 29: 168-191. https://goo.gl/jQEXp3
- Feynman RP. There’s plenty of room at the bottom. Presentation to the American Physical Society. 1959
- Poon AS, O’Driscoll S, Meng TH. Optimal operating frequency in wireless power transmission for implantable devices. Conf Proc IEEE Eng Med Biol Soc. 2007: 5674-5679.
- Poon A, O’Driscoll, Meng TH. Optimal frequency for wireless power transmission in to dispersive tissue. IEEE Trans Antennas Propag. 2010; 58: 1739-1750. https://goo.gl/CJkNn1
- Tyler Perryman L, Larson P, Glaser J. Tissue depth study for a fully implantable, remotely powered and programmable wireless neural stimulator. Int J Nano Stud Technol. 2016; S2: 001: 1-6. https://goo.gl/Fz6MMf
- Weiner RL, Garcia CM, Vanquathem N. A novel miniature wireless neurostimulator in the management of chronic craniofacial pain: preliminary results from a prospective pilot study. Scand J Pain. 2017; 17: 350-354. https://goo.gl/kjNT4k
- Perryman LT, Speck B, Weiner RL. A novel wireless minimally invasive neuromodulation device for the treatment of chronic intractable occipital neuralgia: case illustrations. J Neurol Stroke. 2017; 6: 00213. https://goo.gl/NjPj2h
- Billet B, Wynendaele R, Vanquathem N. A novel minimally invasive wireless technology for neuromodulation via Percutaneous Intercostal Nerve Stimulation (PNS) for post-herpetic neuralgia: a case report with short term follow up. Pain Pract. 2018; 18: 374-379. https://goo.gl/awsBwk
- Herschkowitz D, Kubias J. Wireless peripheral nerve stimulation for complex regional pain syndrome type I of the upper extremity: a case illustration introducing a novel technology. Scand J Pain. 2018; 18: 555-560. https://goo.gl/GczN4M

Sign up for Article Alerts